Research and Development in the Pharmaceutical Industry
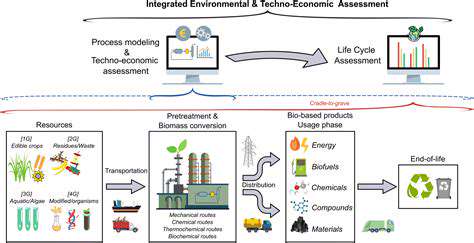
In the pharmaceutical sector, research and development (R&D) serves as the backbone of medical breakthroughs. It drives the creation of novel treatments and cures, transforming how we manage illnesses. This endeavor demands substantial capital and relentless investigative work. Bringing new medications to market isn't simple—it requires seamless teamwork among scientists, investigators, and medical practitioners.
The journey from initial concept to pharmacy shelves spans years of meticulous work. Drug development involves multiple checkpoints: discovery, testing, and regulatory hurdles. Breakthroughs in R&D literally save lives, particularly in battling complex conditions like malignancies and viral outbreaks. Each phase demands precision to ensure patient safety and treatment efficacy.
The Critical Role of Clinical Trials
Human trials form the cornerstone of pharmaceutical progress. These carefully structured studies evaluate new medications' performance and safety in real patients. Researchers design these trials with rigorous protocols to guarantee data accuracy. Ethical practices remain non-negotiable throughout this process.
Clinical testing occurs in sequential phases, each serving distinct purposes. Early phases focus on safety, while later stages examine effectiveness across larger groups. These findings determine whether experimental treatments advance toward approval. Trial data also helps customize therapies for specific patient demographics.
Financial and Technological Investments
Pharmaceutical innovation requires massive funding. While corporations and investors provide significant capital, government grants also fuel critical research. Public funds often target pressing medical challenges lacking commercial incentives.
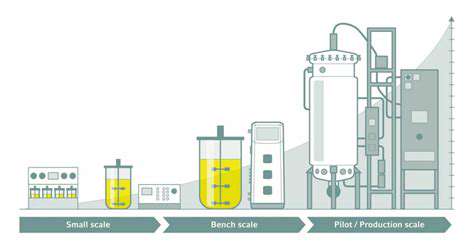
State-of-the-art laboratories and equipment accelerate discovery timelines. Advanced facilities empower researchers to conduct sophisticated experiments, shortening development cycles. Maintaining these resources remains essential for sustained innovation in drug development.
Navigating Regulatory Requirements
Government agencies enforce strict rules governing new medications. These safeguards protect consumers while ensuring drugs perform as intended. Pharmaceutical firms must demonstrate compliance throughout development.
Regulatory reviews involve exhaustive documentation and analysis. Approval hinges on proving a treatment's benefits outweigh potential risks. This thorough vetting process often spans years, underscoring the importance of meticulous research practices.
The Power of Collaborative Innovation
No single organization possesses all necessary expertise. That's why partnerships between companies, universities, and research centers prove invaluable. Shared knowledge propels discoveries forward faster than isolated efforts.
These alliances take various forms—from joint studies to resource pooling. Strategic collaborations frequently yield comprehensive solutions that enhance patient care globally. Such cooperative models represent the future of medical advancement.
Marketing and Sales: Connecting Innovation with the Market
Bridging Science and Market Needs
Effective biotech marketing begins with understanding end-user requirements. Companies must articulate how their innovations solve real problems for healthcare providers and patients. This requires identifying target demographics, crafting compelling stories about research impacts, and deploying multi-channel engagement strategies. Market intelligence and regulatory awareness help tailor approaches for maximum effectiveness.
Beyond transactions, successful firms cultivate lasting relationships. They earn trust through ongoing support, scientific updates, and responsive service. This customer-centric approach fosters loyalty and repeat business in this relationship-driven sector.
Cultivating Brand Authority
Distinct brand positioning is critical in biotech. Companies must clearly communicate their scientific rigor, ethical standards, and innovation capabilities. Consistency across all touchpoints—from investor presentations to product literature—builds credibility over time.
Demonstrating technical expertise through white papers and conference participation reinforces market positioning. Transparency about research methodologies strengthens stakeholder confidence in the company's offerings and long-term viability.
Regulatory Strategy and Market Access
Biotech marketers must master complex compliance requirements. They need in-depth knowledge of approval processes across different jurisdictions to avoid costly delays. This expertise informs go-to-market timing and messaging strategies.
Effective market entry requires nuanced understanding of regional differences. Localized approaches addressing regulatory and cultural variations optimize commercial success. Companies often employ phased launches to manage risk and resource allocation.
Relationship-Centric Sales Approach
Biotech sales success hinges on deep customer understanding. Representatives must grasp clients' operational challenges and strategic goals. Solutions-oriented dialogues outperform traditional sales pitches in this technical field.
Post-sale engagement differentiates market leaders. Providing ongoing education, troubleshooting support, and research updates transforms transactions into partnerships. This investment in relationships generates referrals and repeat business.
Data-Driven Commercial Strategy
Analytics transform raw information into competitive advantage. Monitoring therapeutic trends, competitor moves, and customer behavior informs product development and messaging. Clinical outcomes data proves particularly valuable for evidence-based marketing.
Segmentation analysis enables precision targeting of high-value customer groups. Ethical data utilization maintains trust while optimizing campaign performance. Continuous feedback loops ensure strategies remain aligned with market evolution.
Regulatory Affairs: Ensuring Safety and Compliance

The Regulatory Backbone of Drug Development
Regulatory professionals serve as crucial gatekeepers in pharma, safeguarding medication quality. Their work spans the entire product lifecycle, ensuring adherence to standards set by agencies like the FDA. This rigorous oversight prevents unsafe products from reaching patients while facilitating access to effective treatments.
From laboratory benches to pharmacy counters, regulatory teams coordinate complex approval processes. They synthesize technical documentation, manage stakeholder communications, and track compliance deadlines. This multifaceted work ensures medicines meet all safety and efficacy requirements.
Foundational Pre-Clinical Work
Animal studies provide initial safety signals before human testing begins. Regulatory specialists ensure these studies follow Good Laboratory Practices, with proper controls and documentation. Quality preclinical data forms the bedrock for subsequent clinical trial approvals. Any shortcomings here can derail years of research and investment.
Overseeing Human Trials
Clinical study management requires balancing scientific rigor with ethical imperatives. Regulatory professionals design protocols that protect participants while generating valid data. They monitor trial conduct, ensuring consistent adherence to approved methodologies across study sites.
Data integrity checks and adverse event reporting fall under their purview. Meticulous attention to detail during trials prevents costly regulatory queries later. Their work ensures collected evidence withstands regulatory scrutiny.
The Approval Marathon
Marketing applications represent the culmination of years of research. Regulatory teams compile thousands of pages documenting every aspect of the drug's development. Chemistry, manufacturing controls, clinical results, and proposed labeling all undergo intense review.
Submission quality directly impacts approval timelines and success rates. Experienced regulatory professionals anticipate reviewer concerns, preemptively addressing potential objections through comprehensive data presentation.
Vigilance Beyond Approval
Post-marketing surveillance represents an ongoing safety net. Regulatory teams monitor real-world usage, investigating any reported adverse effects. They implement risk mitigation strategies when needed, from label updates to market withdrawals.
This continuous monitoring protects public health as products reach diverse patient populations. It also provides valuable feedback for improving future drug development efforts.
Global Compliance Challenges
Operating across borders introduces regulatory complexity. Each market maintains unique requirements for clinical data, manufacturing standards, and labeling. Regulatory professionals navigate these differences, sometimes running parallel submission processes.
Cultural and legal variations demand flexible yet compliant approaches. Successful global registrations require deep understanding of regional regulatory philosophies and decision-making processes.