In modern biotechnology, cellular factories represent a groundbreaking approach to manufacturing valuable compounds. These biological systems are engineered to synthesize target molecules with precision, often outperforming conventional production techniques. The key lies in reprogramming natural cellular mechanisms to serve specialized functions. Researchers achieve this by meticulously adjusting genetic networks to optimize output while minimizing unnecessary byproducts.
At its core, this methodology involves deciphering cellular operations and strategically modifying them through genetic intervention. This paradigm shift enables scientists to transcend natural biological constraints, creating organisms specifically tailored for large-scale production of pharmaceuticals, biofuels, and other complex compounds.
Genetic Engineering for Enhanced Productivity
Genetic modification serves as the cornerstone of cellular optimization. Through precise gene editing, researchers can introduce novel genetic material, adjust existing sequences, or eliminate unnecessary genes to reshape metabolic processes. Such targeted alterations permit exact control over cellular functions, transforming microorganisms into efficient production units that convert substrates into desired end products. Enzyme engineering further enhances this process by improving catalytic efficiency or expanding operational parameters.
This precision-oriented genetic approach yields highly specialized cellular systems designed for specific manufacturing requirements. The resulting biofactories demonstrate remarkable efficiency, converting raw materials into valuable products with minimal resource waste.
Metabolic Engineering for Optimized Pathways
Metabolic pathway optimization focuses on streamlining intracellular processes to maximize output of target molecules. This requires identifying critical enzymes, regulatory points, and metabolic bottlenecks within pathways. Strategic genetic modifications address these limitations, significantly boosting overall production efficiency. Interventions may include introducing novel enzymes, adjusting enzymatic activity levels, or completely redesigning metabolic routes to establish more efficient material flows.
Strain Development and Optimization for Scalability
For industrial applications, developing robust microbial strains is paramount. Selection and refinement processes create microorganisms capable of high-yield production across diverse environmental conditions. Optimization strategies encompass adaptation to varying temperatures, nutrient concentrations, and growth media formulations. Such refinement ensures consistent, reliable performance even in large-scale bioreactor environments.
Process Optimization and Bioreactor Design
Comprehensive process optimization extends beyond cellular modifications to include production environment design. Bioreactors must provide ideal conditions for cellular growth and product synthesis. Critical parameters like temperature, pH, oxygenation, and nutrient supply require meticulous control to maximize output while preventing contamination. Continuous monitoring systems and adaptive feedback mechanisms maintain optimal conditions throughout the production cycle.
Cost-Effectiveness and Sustainability Considerations
Economic viability and environmental sustainability form crucial aspects of cellular factory implementation. Strategies focus on minimizing expensive inputs, reducing energy consumption, and optimizing waste management. Developing sustainable raw material sources and efficient purification methods is essential for large-scale feasibility. Comprehensive environmental impact assessments must accompany technological development to ensure responsible implementation.
Ethical and Safety Considerations in Synthetic Biology
The powerful potential of synthetic biology necessitates rigorous ethical and safety protocols. Potential risks, including unintended ecological consequences or accidental organism release, require thorough evaluation. Robust safety measures and regulatory frameworks must govern development and application of these technologies. Transparent public discourse ensures responsible, ethical utilization of synthetic biology advancements.
Tailoring Metabolic Pathways for Desired Outputs
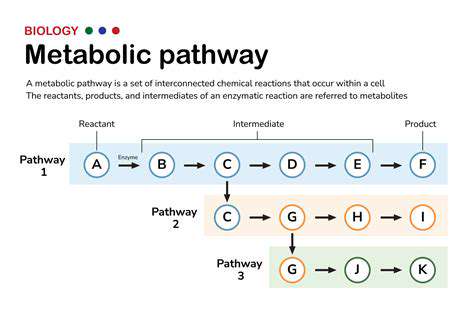
Metabolic Engineering Strategies
Metabolic pathway engineering represents a transformative approach in modern biotechnology. This methodology focuses on precisely adjusting cellular metabolic networks to achieve specific manufacturing objectives. Applications range from pharmaceutical production to biofuel generation, all accomplished by strategically redirecting cellular resources. Successful implementation demands comprehensive understanding of intricate intracellular networks, including enzymatic processes, transport mechanisms, and regulatory systems.
Critical to this approach is identifying pivotal enzymes that govern metabolic flux. Modifying these key catalysts through genetic intervention can dramatically influence pathway output. Advanced techniques including gene silencing, overexpression, and heterologous gene introduction enable precise metabolic control, resulting in enhanced efficiency and yield improvements.
Pathways and their Interactions
Effective metabolic engineering requires appreciation of pathway interconnectivity. Understanding how metabolic routes share intermediates and respond to cellular signals informs strategic intervention points. This comprehensive perspective prevents unintended consequences while maximizing desired outcomes. Pathway modifications often create ripple effects throughout cellular metabolism, necessitating holistic system analysis.
Cellular metabolic networks function as integrated systems rather than isolated pathways. Production of specific metabolites frequently requires redirecting intermediates from other processes. Accounting for these complex interactions enables engineers to maximize target output while maintaining cellular equilibrium. This systems-level understanding proves essential for navigating metabolic complexity.
Targeting Specific Enzymes
Strategic enzyme targeting forms the foundation of effective metabolic engineering. Key catalytic proteins controlling metabolic flux represent prime intervention points. Focused modification of these enzymes permits precise metabolic pathway control, leading to enhanced efficiency and productivity. Rate-limiting enzymes particularly influence overall pathway output when modified.
Advanced protein engineering methods including directed evolution and rational design enable fine-tuning of enzymatic properties. These techniques yield optimized catalysts with improved performance characteristics, directly increasing valuable metabolite production.
Computational Modeling and Optimization
Computer-aided modeling provides indispensable support for metabolic engineering efforts. Sophisticated simulations incorporating enzyme kinetics and cellular conditions allow researchers to predict modification outcomes before implementation. This predictive capacity facilitates optimal strategy development while avoiding potential pitfalls. Advanced algorithms identify ideal genetic modification combinations to maximize metabolic pathway efficiency.
These computational tools significantly reduce development time and resource expenditure, accelerating the creation of optimized production systems. Such approaches prove invaluable for bioreactor design and industrial process improvement.
Advanced Genetic Tools and Design Principles
Gene Editing Technologies: Precision Tools for Modification
Modern genetic engineering benefits from revolutionary tools like CRISPR-Cas9, enabling unprecedented DNA sequence precision. These systems facilitate accurate gene modifications, deletions, or insertions, proving invaluable for synthetic biology applications. While CRISPR dominates current research, alternative technologies including TALENs and ZFNs offer complementary approaches, providing researchers with diverse options for genetic manipulation.
Synthetic Promoters and Terminators: Controlling Gene Expression
Precise gene expression control forms a critical component of synthetic biology. Custom-designed regulatory elements enable fine-tuned control over transcription levels and timing. These engineered components prove essential for metabolic pathway control and protein production optimization.
Metabolic Engineering for Enhanced Pathway Design
Metabolic pathway redesign represents a central focus of synthetic biology applications. Computational modeling supports this process, enabling efficient pathway design that maximizes output. The ultimate goal involves creating sustainable, high-yield bioproduction systems for industrial implementation.
Modular Design Principles: Building Blocks for Complex Systems
Complex biological systems benefit from modular construction approaches. Dividing systems into functional components simplifies design and testing processes. This methodology facilitates stepwise development of sophisticated production systems through discrete module optimization.
Computational Modeling and Simulation: Predicting System Behavior
Computer simulations provide critical predictive capability for synthetic biology. These tools enable researchers to model complex biological systems before physical implementation, identifying optimization opportunities and potential issues. Such predictive modeling significantly accelerates bioproduction system development.
Strain Optimization for Enhanced Performance
Host organism optimization represents a crucial aspect of bioproduction. Genetic modifications enhance microbial productivity, stability, and resilience. These improvements maximize output while minimizing undesirable byproducts, creating robust production platforms.
Quality Control and Process Optimization: Ensuring Reliable Outcomes
Stringent quality measures ensure consistent, high-quality bioproduction output. Comprehensive monitoring of purity, yield, and consistency maintains product standards. Process optimization simultaneously enhances efficiency and productivity, creating streamlined manufacturing systems.
Scaling Up Biomanufacturing Processes
Optimizing Bioreactor Design for Enhanced Productivity
Bioreactor engineering significantly impacts bioproduction efficiency. Critical design considerations include mixing dynamics, thermal regulation, and gas exchange parameters, all influencing microbial performance. Various reactor configurations—including stirred-tank, airlift, and packed-bed designs—offer distinct advantages for specific applications. Impeller selection and operational parameters require careful optimization to balance mixing efficiency with cellular integrity.
Oxygen transfer efficiency proves particularly crucial for aerobic bioprocesses, directly affecting microbial metabolism and growth kinetics. Optimizing gas-liquid interface parameters, oxygen solubility, and agitation rates can dramatically improve overall process productivity. These optimizations form the foundation for effective downstream processing.
Streamlining Downstream Processing for Cost-Effective Product Recovery
Post-bioreactor processing represents a critical production phase involving product isolation, purification, and formulation. Efficient separation techniques are essential for achieving commercial viability while maintaining product quality. Multi-stage processing typically includes cell disruption, separation, and purification steps, each requiring specific optimization.
Separation technology selection directly impacts process economics and scalability. Centrifugation, filtration, and chromatography each serve distinct roles in product recovery. Strategic combination of these methods, coupled with parameter optimization, enables cost-effective manufacturing. Integrated quality control measures ensure consistent product specifications and regulatory compliance throughout processing stages.