CRISPR-Cas9 technology, a revolutionary gene-editing tool, is rapidly transforming the field of neuroscience and holds immense promise for treating neurological disorders. This system, derived from bacterial immune systems, allows researchers to precisely target and modify specific DNA sequences within the genome. This precision is crucial for correcting genetic mutations that cause diseases like Huntington's disease, muscular dystrophy, and certain forms of epilepsy. The potential for targeted gene correction in human cells opens doors for developing novel therapies to combat these devastating conditions, offering hope for patients previously thought to have limited treatment options.
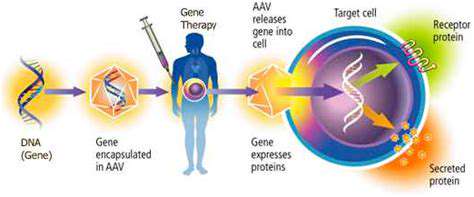
The mechanism of CRISPR-Cas9 involves guiding the Cas9 enzyme to a specific location in the genome using a short RNA molecule. Once there, the enzyme cuts the DNA, allowing for the introduction of new genetic material or the removal of faulty segments. This precise editing capability enables scientists to address the underlying genetic causes of neurological disorders, potentially leading to more effective and personalized treatments. The ongoing research and development in this area are crucial for translating this powerful technology into tangible clinical benefits for patients.
Beyond CRISPR: Exploring Alternative Gene Editing Approaches
While CRISPR-Cas9 has garnered significant attention, several other gene editing technologies are also emerging as potential therapeutic avenues for neurological diseases. Zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) are examples of earlier gene editing tools that have paved the way for CRISPR. Each method has its own advantages and limitations in terms of targeting specificity, efficiency, and delivery systems. Researchers are actively investigating these alternatives to identify the most suitable approaches for specific neurological conditions, considering factors like the complexity of the genetic defects and the feasibility of delivery to the target tissues in the brain.
One important aspect of exploring alternative gene editing technologies is the development of more efficient and targeted delivery mechanisms. Delivering these tools directly to the affected neurons or brain regions is crucial for maximizing therapeutic efficacy. Novel approaches, such as viral vectors and nanocarriers, are being investigated to ensure that the gene-editing tools reach their intended targets within the nervous system with minimal off-target effects. This research is critical for improving the safety and effectiveness of these potentially transformative therapies.
Clinical Translation and Ethical Considerations
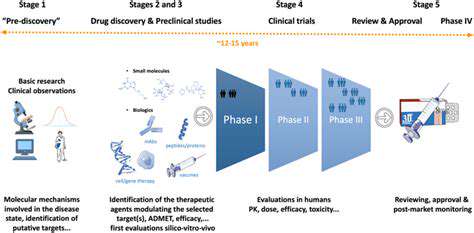
The successful translation of gene editing technologies from the laboratory to clinical settings is a significant hurdle. Rigorous preclinical testing in animal models is essential to demonstrate safety and efficacy before initiating human trials. Researchers must carefully assess potential off-target effects, immune responses, and long-term consequences of gene editing interventions. This cautious approach is vital to minimize risks and ensure the responsible development of these groundbreaking therapies.
Furthermore, ethical considerations surrounding gene editing technologies require careful consideration. The potential for germline editing, which modifies genes that are passed on to future generations, raises complex ethical dilemmas. Open discussions and robust regulatory frameworks are necessary to ensure that gene editing technologies are used responsibly and ethically. Public engagement and a thorough understanding of the potential benefits and risks are essential for guiding the responsible development and implementation of these transformative therapies for neurological disorders.
The ongoing research and development in gene editing technologies are paving the way for a future where previously untreatable neurological disorders could be targeted with precision and potentially cured. This holds immense promise for improving the lives of countless individuals and families affected by these conditions, but also necessitates careful consideration of the ethical and practical implications.

The Future of Gene Editing in Neurological Development: A Multifaceted Approach

Gene Editing for Neurological Disorders: A Promising Future
Gene editing technologies, particularly CRISPR-Cas9, are rapidly evolving, offering exciting possibilities for treating a wide range of neurological disorders. These advancements hold the potential to revolutionize the way we approach these debilitating conditions, offering targeted therapies that could correct genetic defects responsible for disease development. Scientists are exploring various avenues to exploit this technology, ranging from correcting mutations causing inherited diseases to manipulating cellular pathways in neurodegenerative conditions. The potential to precisely target and modify specific genes within the nervous system is opening up a new frontier in medicine, promising a future where previously untreatable conditions could be effectively addressed.
The development of gene editing tools is not without challenges. One key area of concern is ensuring the safety and efficacy of these therapies. Extensive preclinical testing is crucial to assess off-target effects and potential long-term consequences of gene editing in the nervous system. Rigorous testing is essential to ensure that these therapies are not only effective but also safe for patients. Furthermore, the complexity of the human nervous system presents unique challenges, necessitating a deep understanding of the intricate cellular and molecular processes involved in neurological disorders. This necessitates advanced research and development in order to translate experimental findings into clinically viable treatments.
Precision Medicine Approaches to Gene Editing
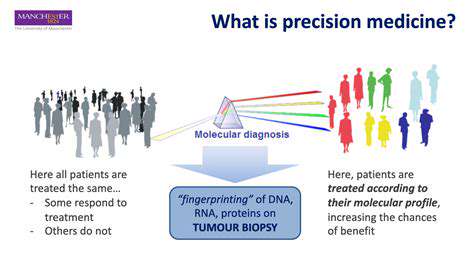
The future of gene editing in neurology is inextricably linked to the concept of precision medicine. This approach aims to tailor treatments to individual patients based on their unique genetic makeup and disease characteristics. Gene editing technologies provide a powerful tool to achieve this personalized approach, enabling the correction of specific genetic mutations that contribute to a patient's condition. Personalized medicine is expected to play a critical role in optimizing gene editing therapies, maximizing efficacy and minimizing adverse events.
By understanding the specific genetic variations that drive a patient's neurological disease, we can develop highly targeted treatments that are more likely to be effective and have fewer side effects. This approach also offers the possibility of preventing the disease from developing in the first place, possibly through preventative gene editing strategies. The ability to precisely target specific cells and pathways within the nervous system is key to the success of this approach. This requires a thorough understanding of the complex biological pathways involved, along with careful consideration of the ethical implications associated with manipulating the human genome.
A crucial aspect of precision medicine is the development of diagnostic tools that can accurately identify the specific genetic mutations responsible for a patient's condition. This information is vital for selecting the appropriate gene editing strategy and monitoring treatment effectiveness. The development of these diagnostic tools is a critical step in the path toward personalized gene editing therapies.