Traditional Drug Discovery: A Time-Consuming Process
Traditional drug discovery methods have long depended on exhaustive trial-and-error experimentation, a method that consumes substantial time and financial resources. Scientists typically begin with extensive libraries of potential compounds, meticulously testing each one against specific proteins or biological pathways. This involves countless hours in the laboratory, painstaking data collection, and repeated iterations before any viable candidate emerges. The massive number of compounds requiring screening, coupled with rigorous validation procedures, results in prolonged development timelines. Such drawn-out processes make it difficult to respond promptly to urgent health emergencies. Moreover, this conventional approach demands exorbitant investments, often spanning over a decade before a drug becomes commercially available.
The Limitations of Traditional High-Throughput Screening
While high-throughput screening (HTS) represents progress over older techniques, it still suffers from notable drawbacks. HTS automates compound testing but remains burdened by the need to sift through vast, often irrelevant chemical libraries. Given the enormity of chemical space, many screened compounds prove unsuitable for the intended targets, resulting in inefficient resource allocation. Additionally, HTS frequently fails to detect compounds with intricate mechanisms or those requiring specific biological environments. These shortcomings underscore the necessity for more precise and intelligent methodologies.
The Rise of Artificial Intelligence in Drug Discovery
The advent of artificial intelligence (AI) is dramatically reshaping drug discovery. Machine learning algorithms excel at processing enormous biological datasets, including genomic sequences, protein structures, and experimental outcomes. This capability enables researchers to pinpoint potential drug candidates with unprecedented accuracy and speed. By leveraging existing data, AI can forecast compound efficacy and safety, potentially revolutionizing lead identification.
Predictive Modeling for Target Identification and Validation
AI-driven predictive models analyze intricate biological networks to uncover novel drug targets. These models predict protein interactions, elucidate disease pathways, and highlight potential therapeutic vulnerabilities. This represents a monumental leap from traditional empirical approaches. By identifying more relevant targets and validating them efficiently, AI-powered tools can drastically reduce the time needed to discover promising drug candidates.
Accelerated Compound Identification and Optimization
AI dramatically speeds up the identification and refinement of potential drug candidates. By evaluating extensive chemical databases and assessing structural properties, activity, and toxicity, AI prioritizes compounds with high success probabilities. Incorporating real-time experimental feedback creates a dynamic optimization loop, continuously improving molecular designs.
Personalized Medicine: AI's Role in Tailoring Treatments
AI isn't just transforming drug discovery; it's pioneering personalized medicine. By analyzing individual patient data, including genetic profiles and lifestyle factors, AI helps customize treatments to specific needs. This tailored approach enhances treatment efficacy while minimizing adverse effects. It's particularly valuable for addressing complex individual responses to various therapies, enabling more precise and effective interventions.
The Future of Drug Discovery: A Collaborative Approach
Future drug discovery will likely integrate AI with traditional methods, combining their respective strengths. AI can enhance conventional techniques by providing critical insights and tools. Such collaboration ensures responsible and ethical AI application while maximizing its potential to advance healthcare. The synergy between human expertise and AI will be crucial for navigating ethical considerations and the complexities of these powerful technologies.
AI's Role in Identifying Novel Drug Targets
AI's Accelerated Drug Discovery Process
Artificial intelligence (AI) is revolutionizing pharmaceuticals, particularly in drug discovery. AI algorithms process massive datasets of molecular structures, biological pathways, and clinical trial results to identify drug candidates far quicker than traditional methods. This acceleration could mean life-saving medications reach patients years sooner, dramatically improving outcomes.
AI's data-processing prowess allows researchers to concentrate on the most promising leads, eliminating dead ends and conserving resources. The resulting efficiency translates to substantial cost reductions, freeing up funds for critical development phases.
Identifying Potential Drug Targets
AI excels at discovering potential drug targets by analyzing complex biological systems. It pinpoints critical molecules or pathways involved in disease progression, offering a more efficient alternative to traditional trial-and-error approaches. This capability could lead to groundbreaking treatments and deeper disease understanding.
Predicting Drug Efficacy and Safety
AI models forecast drug efficacy and safety before laboratory testing begins. This predictive capability helps avoid pursuing ineffective or hazardous candidates, significantly mitigating clinical trial risks. Early risk identification steers researchers toward safer, more effective development strategies, saving both time and resources while ensuring patient safety.
Analyzing and Interpreting Experimental Data
AI automates the analysis of complex experimental data like genomic sequencing and protein interactions. This automation allows researchers to focus on higher-level tasks such as experimental design and result interpretation. AI's ability to detect patterns imperceptible to humans is invaluable for uncovering crucial insights.
Personalized Medicine and AI
AI promises to transform personalized medicine by customizing treatments based on individual genetics and disease characteristics. This approach could maximize treatment effectiveness while minimizing side effects. By analyzing patient-specific data, AI identifies optimal therapies and predicts individual responses, ushering in an era of more precise and safer treatments.
Predicting Drug Efficacy and Safety: AI-Driven Simulations
Predicting Drug Efficacy
Accurate efficacy prediction is vital for successful drug development. It requires understanding drug-target interactions, considering chemical structure, solubility, and metabolic pathways. Predictive models using computational simulations can drastically reduce the time and resources needed to identify viable candidates. Sophisticated algorithms and extensive drug databases help discern patterns that predict novel compound efficacy before costly experimental testing.
Understanding Drug Targets
Identifying precise molecular targets is fundamental to efficacy prediction. Thorough knowledge of target structure and function enables the design of drugs that selectively bind and modulate activity, minimizing side effects by acting only on intended pathways.
Computational Modeling
Computational models simulate drug-target interactions, predicting binding affinity and potential efficacy. These simulations offer crucial insights into mechanisms of action and possible side effects before expensive clinical trials begin.
In Silico Drug Screening
In silico screening rapidly evaluates numerous potential drug candidates computationally. This approach eliminates early-stage laboratory experiments, significantly accelerating development. It's particularly effective for identifying molecules with high target protein affinity.
Predicting Drug Metabolism and Pharmacokinetics
Understanding a drug's absorption, distribution, metabolism, and excretion (ADME) is crucial for predicting its pharmacokinetics. Predictive models help determine bioavailability, half-life, and potential drug interactions.
Assessing Drug Safety
Safety prediction is as critical as efficacy assessment. Computational methods simulate drug interactions with biological systems to identify potential adverse effects early. This early detection reduces clinical trial risks and enhances patient safety.
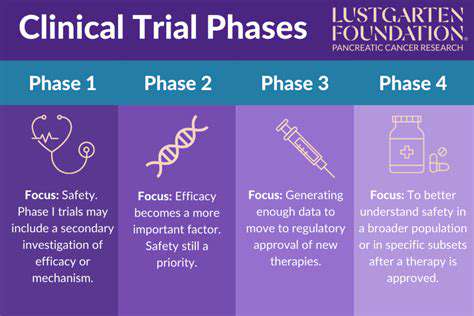
Clinical Trial Design
Predictive modeling informs clinical trial design by identifying likely responders and potential adverse effects. This targeted approach optimizes resource use and minimizes negative outcomes, yielding better safety and efficacy understanding.
Human activities are the primary drivers of greenhouse gas emissions, which play a major role in global climate change. These emissions consist of gases like carbon dioxide, methane, and nitrous oxide that trap heat in the atmosphere, causing a gradual increase in temperatures. This warming effect represents a serious challenge for ecosystems and societies across the globe.