- Regulate gene activity without altering DNA sequences
- Disable harmful genes causing cancer or viral infections
- Introduce therapeutic genes to specific cell types
This versatility makes CRISPR invaluable for both treatment and research, accelerating our understanding of genetic mechanisms and disease pathways.
Navigating Ethical Complexities
With great power comes significant responsibility. Germline editing raises profound ethical questions about altering inheritable traits and the potential for unintended generational consequences. The scientific community has established strict guidelines prohibiting reproductive use of edited embryos while research continues.
Public engagement remains crucial as we develop appropriate regulations. The intersection of genetics and big data adds another layer of complexity to these discussions.
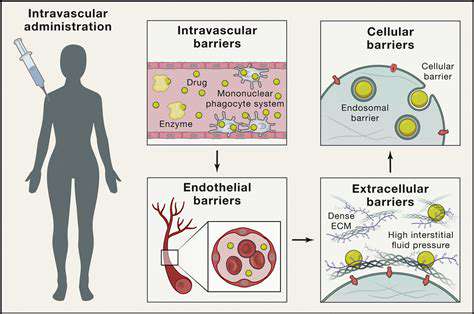
Optimizing Delivery Systems
Therapeutic success depends on getting CRISPR components to the right cells. Current approaches include:
- Viral vectors (modified viruses)
- Lipid nanoparticles
- Physical delivery methods
Each method has advantages for different tissues, with ongoing research improving targeting precision and reducing immune reactions.
Clinical Progress and Cautious Optimism
Early human trials demonstrate CRISPR's potential while revealing areas needing refinement. Current studies focus on:
- Blood disorders (sickle cell, beta thalassemia)
- Eye diseases (Leber congenital amaurosis)
- Certain cancers (CAR-T cell therapies)
These pioneering efforts will shape the future landscape of genetic medicine.
Public Understanding and Acceptance
Building trust requires transparent communication about both capabilities and limitations. Misconceptions about designer babies often overshadow CRISPR's legitimate therapeutic potential. Educational initiatives must emphasize:
- Current medical applications
- Stringent ethical guidelines
- Regulatory oversight processes
Beyond CRISPR: Alternative Gene Editing Approaches
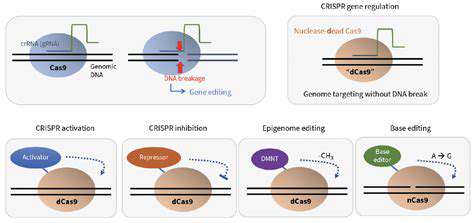
Exploring the Gene Editing Toolkit
While CRISPR dominates headlines, researchers continue developing alternative methods for specific applications. Each technology offers unique advantages depending on the editing requirements.
Zinc Finger Nucleases (ZFNs)
Among the first programmable gene editors, ZFNs demonstrated the feasibility of targeted DNA modification. These engineered proteins combine DNA-binding zinc finger domains with nuclease enzymes. Though less flexible than CRISPR, ZFNs remain valuable for:
- Validating CRISPR results
- Applications requiring protein-based targeting
TALENs: Precision Protein Tools
Transcription activator-like effector nucleases improved upon ZFN technology with:
- Simpler design rules
- Increased targeting flexibility
While powerful, both ZFNs and TALENs require custom protein engineering for each target, limiting widespread adoption.
Meganucleases
These naturally occurring enzymes recognize long DNA sequences (12-40 base pairs), offering exceptional specificity. Their precision comes at the cost of limited target options, as each meganuclease recognizes only one sequence. Current research focuses on engineering variants with expanded targeting capabilities.
Base Editing
This revolutionary approach enables single-letter DNA changes without creating double-strand breaks. Base editors are particularly valuable for correcting point mutations that account for many genetic disorders. The technology combines CRISPR targeting with enzyme domains that chemically convert one base to another.
Prime Editing
Building on base editing, prime editors can perform all possible base changes plus small insertions and deletions. This search-and-replace system offers:
- Greater editing versatility
- Reduced DNA damage
- Higher precision
Early results suggest potential for correcting nearly 90% of known pathogenic variants.
Gene Drives
These controversial systems ensure edited genes spread through populations. Potential applications include:
- Eradicating mosquito-borne diseases
- Controlling invasive species
Ecological concerns and strict regulations currently limit their use to laboratory research.
Challenges and Ethical Considerations
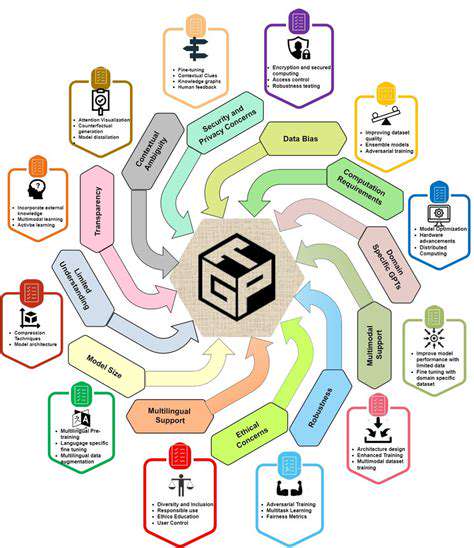
Data Privacy in Genetic Medicine
The intersection of genomics and AI raises significant privacy concerns as genetic data represents the ultimate personal identifier. Effective safeguards must address:
- Secure storage of genomic data
- Consent for secondary research uses
- Protection against discrimination
Algorithmic Bias in Genetic Analysis
Machine learning tools analyzing genetic data may inherit biases from:
- Underrepresented populations in research
- Historical data collection practices
Diverse reference genomes and rigorous validation are essential for equitable precision medicine.
Transparency in AI-Assisted Diagnostics
As AI tools assist in genetic interpretation, clinicians and patients need:
- Clear explanations of results
- Understanding of confidence levels
- Visibility into data sources
This transparency builds trust in emerging technologies.
Workforce Evolution
Gene editing advancements will transform healthcare jobs, requiring:
- Specialized genetic counselors
- Bioinformatics experts
- Ethics specialists
Educational systems must adapt to prepare the next generation workforce.
Security in the Genetic Age
Protecting genetic databases from cyber threats is paramount because:
- Genetic information cannot be reset like passwords
- Breaches could enable sophisticated bio-targeting
Multilayered security approaches are essential.
Legal Responsibility Frameworks
As therapies combine gene editing with AI analysis, liability questions emerge regarding:
- Unanticipated treatment outcomes
- Algorithmic interpretation errors
Clear legal standards must evolve alongside the technology.
Societal Impacts
Widespread gene editing could affect:
- Health disparities
- Cultural perceptions of disability
- Evolutionary trajectories
Ongoing ethical analysis must guide these profound changes.
Clinical Trials and Future Directions
Current Trial Landscape
Over 50 CRISPR-based clinical trials are underway globally, primarily focusing on:
- Hematologic disorders
- Ophthalmologic conditions
- Cancer immunotherapies
Early results demonstrate proof-of-concept while highlighting areas for improvement.
Regulatory Pathways
Gene therapies face unique regulatory challenges including:
- Long-term monitoring requirements
- Manufacturing complexity
- Novel safety considerations
Regulators are developing specialized frameworks for these advanced therapies.
Preclinical Innovation
Animal models continue providing critical insights into:
- Delivery optimization
- Dosing strategies
- Long-term effects
Humanized models better predict therapeutic responses.
Delivery Breakthroughs
Next-generation delivery systems aim to:
- Target specific tissues
- Minimize immune reactions
- Enable repeat dosing
Nanotechnology shows particular promise for crossing biological barriers.
Enhanced Specificity
New CRISPR variants and companion technologies reduce off-target effects through:
- Improved guide RNA design
- High-fidelity Cas enzymes
- Advanced screening methods
These advances increase therapeutic safety margins.
Longitudinal Monitoring
Post-treatment surveillance programs track:
- Therapeutic persistence
- Late-onset effects
- Generational impacts
Such data will shape future clinical guidelines.