Introduction to Adaptive Trial Designs
Understanding the Need for Adaptive Designs
Adaptive clinical trial designs represent a significant advancement in the field of medical research. Traditional fixed designs, while offering a structured approach, often fall short in efficiently exploring the potential of new treatments. These trials typically rely on pre-determined sample sizes and treatment assignments, which can lead to wasted resources if the initial data suggests a different treatment path. Adaptive designs, in contrast, allow for modifications to the trial protocol based on accumulating data, thereby enhancing the likelihood of identifying effective treatments more quickly and efficiently. This flexibility is particularly crucial in situations where the initial hypotheses might not be fully validated or where new information emerges during the trial that warrants a change in strategy.
One of the key reasons for adopting adaptive trial designs is the potential to reduce the time and cost of drug development. By dynamically adjusting the trial as it progresses, researchers can avoid unnecessary testing of ineffective treatments and concentrate resources on promising ones. This agile approach not only accelerates the process but also minimizes the risks associated with pursuing treatments that ultimately prove to be less beneficial. Furthermore, adaptive trials can offer a more refined understanding of treatment effects, allowing for a deeper analysis of subgroups within the study population and enabling the exploration of potential interactions between treatments and patient characteristics.
Key Features and Mechanisms of Adaptive Designs
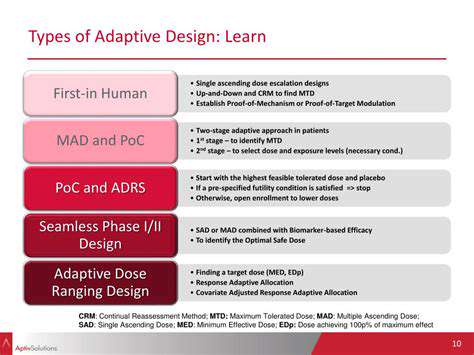
Adaptive designs utilize various mechanisms to achieve their dynamic nature. One common technique is sample size re-estimation, where the initial sample size calculation can be adjusted based on the accumulating data. This allows for potentially more precise estimation of treatment effects with a more optimized sample size, reducing the need for excessive recruitment. Another critical feature is the ability to add or remove treatment arms, allowing the trial to incorporate new treatments or exclude less promising ones based on emerging evidence. This flexibility is vital in a dynamic landscape of medical research, where new insights and discoveries constantly reshape our understanding of diseases and treatments. A key component is also the ability to modify the eligibility criteria, allowing for the inclusion or exclusion of specific patient populations as the trial progresses, potentially refining the study's target population and enhancing the generalizability of results.
Beyond these core mechanisms, adaptive designs can incorporate other strategies, such as modifying the randomization procedures or adjusting the outcome measures. These adjustments can be based on a variety of factors, including the rate of treatment success, the safety profile of different interventions, and the overall feasibility of continuing certain procedures. The key is that these adjustments are carefully planned and implemented in a way that maintains the integrity and validity of the research findings.
Advantages and Considerations of Adaptive Designs
Adaptive clinical trial designs offer several compelling advantages over traditional fixed designs. These include enhanced efficiency in identifying effective treatments, potentially reducing the time and cost of drug development, and an improved ability to capture nuanced information about treatment effects. By allowing for modifications based on emerging data, adaptive designs potentially minimize the risk of pursuing treatments that are ultimately less beneficial. In addition to these benefits, careful consideration must be given to the potential challenges. Rigorous oversight is essential to ensure that the modifications are scientifically justifiable and do not compromise the integrity of the study. This includes the pre-specification of the adaptive rules and the use of statistical methods that account for the flexibility inherent in the design. Furthermore, the complexity of adaptive designs requires specialized expertise in both statistics and clinical research.
Careful planning, transparent reporting, and rigorous statistical analyses are crucial for ensuring the validity and reliability of findings from adaptive trials. This meticulous approach ensures that the benefits of adaptive designs are realized without compromising the integrity of the scientific process. The potential for bias and the need for meticulous monitoring during the trial are important considerations for implementing these designs, emphasizing the need for robust methodological frameworks.

Benefits of Adaptive Clinical Trial Designs
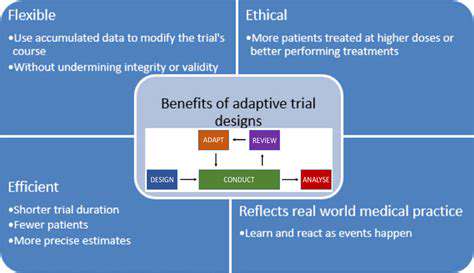
Enhanced Patient Recruitment and Retention
Adaptive clinical trials offer a significant advantage in patient recruitment by allowing researchers to modify the trial design based on accumulating data. This flexibility enables a more targeted approach, attracting patients who are more likely to benefit from the intervention. This targeted approach can lead to more efficient use of resources and a faster time to results. Furthermore, the ability to adapt the trial design can also enhance patient retention rates. Patients may be more engaged and motivated to remain in a study that is tailored to their specific needs and characteristics, leading to a higher completion rate.
Improved Trial Efficiency and Cost-Effectiveness
Adaptive designs can significantly improve the efficiency of clinical trials. By adjusting the trial parameters based on emerging data, researchers can often avoid unnecessary or redundant steps. This streamlined approach can significantly reduce the duration of the trial and the overall costs associated with it. Furthermore, the use of adaptive methods often results in a more focused study population, leading to a greater likelihood of generating statistically significant results from a smaller sample size.
Increased Statistical Power and Precision
Adaptive designs are particularly powerful in maximizing the statistical power of a trial. By dynamically adjusting the sample size and allocation of participants to treatment arms, researchers can ensure that the trial has sufficient statistical power to detect meaningful treatment effects. This precision is crucial in evaluating the effectiveness of an intervention and in drawing reliable conclusions.
Greater Flexibility and Responsiveness to Emerging Data
Adaptive clinical trials offer unmatched flexibility compared to traditional fixed-design trials. The ability to modify the trial protocol in response to emerging data is a critical advantage, particularly in situations where there is uncertainty about the efficacy or safety of the intervention. This responsiveness is essential in modern medicine where new knowledge and insights are constantly evolving. Researchers can quickly adapt to unforeseen circumstances or unexpected findings, ensuring that the trial remains relevant and informative.
Reduced Risk of Bias and Increased Validity
Adaptive designs can help mitigate various sources of bias that may affect the validity of the results in traditional clinical trials. By dynamically adjusting the trial design based on accumulated data, researchers can minimize the impact of confounding factors and biases. This adjustment ensures the trial population is more representative of the target population, leading to more accurate conclusions about the intervention. This enhanced validity is essential for ensuring the reliable application of the findings in clinical practice.
Potential to Improve Patient Outcomes
Ultimately, adaptive trials are designed to improve patient outcomes. By tailoring the trial to emerging data, researchers can ensure the trial is designed to study the most promising interventions and populations. This leads to more efficient use of resources and a greater chance of identifying treatments that are truly effective. By being responsive to the evolving landscape of medical knowledge, adaptive trials have the potential to accelerate the development and implementation of life-saving interventions. The enhanced flexibility of adaptive trials can lead to improved patient outcomes by allowing researchers to respond to emerging information throughout the study, ensuring the trial's relevance and the patients' benefit.