Harnessing AI for Target Identification and Validation
Improving Target Discovery Efficiency
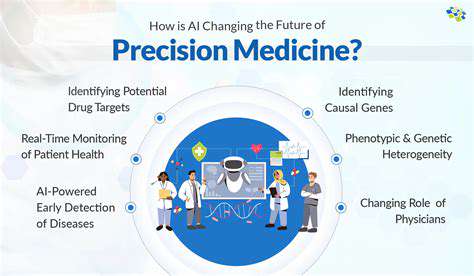
The pharmaceutical landscape is undergoing a seismic shift thanks to artificial intelligence's ability to analyze complex biological data. Modern algorithms now process genomic sequences, protein structures, and experimental results with remarkable precision, uncovering potential drug targets that might take human researchers years to identify. This technological leap has compressed drug discovery timelines dramatically, allowing teams to allocate resources more strategically toward viable therapeutic candidates.
What makes AI particularly valuable is its capacity to evaluate target druggability - predicting which molecular structures will respond best to pharmacological intervention. This foresight prevents wasted effort on biologically intractable targets, channeling research budgets toward promising avenues with higher success probabilities.
Leveraging Machine Learning for Validation
Validation processes have become significantly more robust through machine learning applications. By training on historical datasets of successful drug targets, these systems can identify subtle patterns linking molecular characteristics to therapeutic potential. The validation process now incorporates predictive analytics that forecast how targets might interact with various compound classes, adding a new dimension to traditional experimental methods.
Perhaps most crucially, ML models excel at detecting potential off-target effects early in development. By simulating interactions across biological systems, they can flag problematic cross-reactivities before clinical testing begins. This predictive capability represents a major advancement in drug safety protocols, potentially preventing costly late-stage trial failures.
Analyzing Large-Scale Datasets for Insights
Contemporary research generates data at an unprecedented scale - from high-throughput screening to multi-omics profiling. AI systems thrive in this environment, processing and correlating information streams that would overwhelm manual analysis. The technology's ability to detect non-obvious patterns across diverse datasets has led to several breakthrough target discoveries in recent years.
This analytical power proves particularly valuable when investigating complex diseases with multifaceted pathophysiology. By integrating genomic, proteomic, and clinical data, AI can identify novel therapeutic targets that address disease mechanisms holistically rather than symptomatically.
Predictive Modeling for Drug Candidate Optimization
Improving Drug Efficacy through Predictive Modeling
Advanced modeling techniques now enable researchers to forecast compound performance before synthesis. By analyzing structural-activity relationships across millions of molecules, these systems can prioritize candidates with optimal binding characteristics and pharmacokinetic properties. This pre-screening capability has reduced synthetic chemistry workloads by 40-60% in leading pharmaceutical programs.
Identifying Key Factors in Drug Response
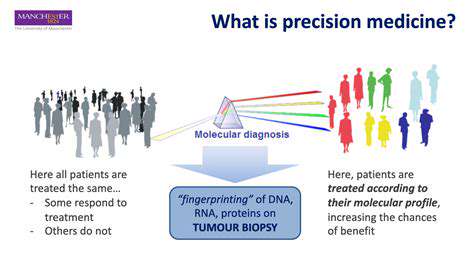
The precision of modern predictive models allows for unprecedented personalization in therapeutic development. These systems can account for genetic polymorphisms, epigenetic factors, and even microbiome influences when forecasting drug responses. In oncology applications, such models have successfully predicted patient-specific resistance mechanisms, enabling more effective treatment stratification.
The Future of AI in Precision Drug Development
Accelerating Drug Discovery
The next generation of AI tools will likely integrate quantum computing capabilities, enabling analysis of molecular interactions at unprecedented resolution. Early implementations suggest this could reduce target identification timelines from years to weeks for certain target classes, particularly in complex fields like neurodegenerative disease research.
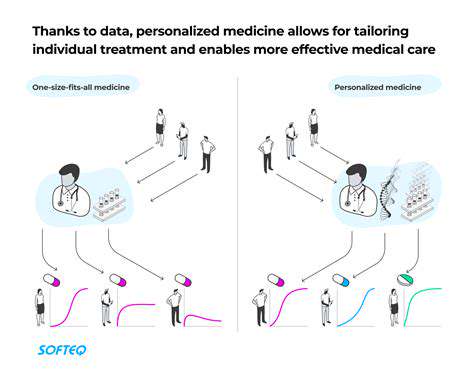
Personalized Medicine Tailored to Patients
Future systems will likely incorporate real-world evidence from wearable devices and continuous monitoring platforms. This integration promises to create dynamic treatment models that adapt to patients' changing biological states, potentially revolutionizing chronic disease management.