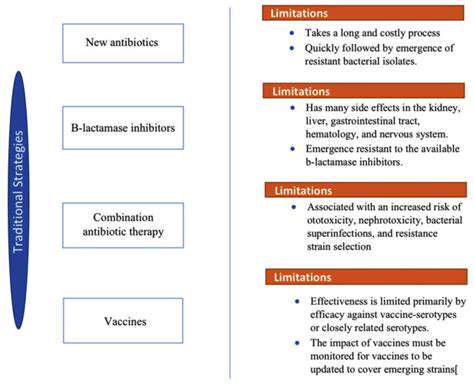
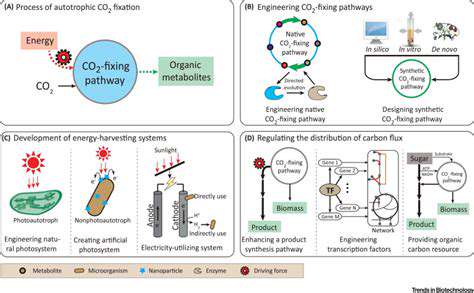
Engineering Microorganisms for CO2 Fixation
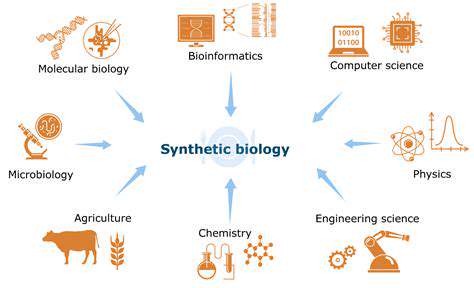
Harnessing Microbial Metabolism
Nature's smallest engineers—microorganisms—hold immense potential for tackling CO2 emissions. These tiny powerhouses can be reprogrammed to treat CO2 as a carbon buffet, transforming it into biofuels and industrial chemicals. Recent breakthroughs show particular promise in adapting these natural processes to reduce fossil fuel dependence while minimizing industrial footprints.
The secret lies in decoding microbial metabolism blueprints. Scientists are mapping the complex biochemical pathways of various microorganisms like explorers charting new territories. By pinpointing key enzymes and regulatory switches for CO2 processing, researchers can design precision genetic upgrades to boost efficiency.
Genetic Engineering Strategies
The CRISPR revolution has opened unprecedented doors for microbial redesign. This molecular scalpel allows surgeons of the microscopic world to edit genes with nucleotide-level precision—adding new capabilities while preserving essential functions. Such surgical modifications can activate dormant metabolic pathways or import superior CO2-processing machinery from other organisms.
Early trials demonstrate staggering potential—some modified strains show 300% increases in CO2 conversion rates. The most successful experiments combine enzymes from extremophile bacteria with industrial microbial workhorses, creating supercharged carbon processors.
Developing Bioreactors
The real test comes in translating lab success to industrial scale. Next-generation bioreactors must function like microbial five-star hotels—maintaining perfect temperature, pH, and nutrient balances while continuously feeding concentrated CO2 streams. Engineers are experimenting with modular designs that can adapt to different microbial communities and processing needs.
Pilot facilities now achieve 90% CO2 capture rates with continuous operation cycles exceeding six months. The most promising designs incorporate self-regulating systems that adjust conditions in real-time based on microbial activity sensors.
Sustainable Production of Biofuels
Microbial refineries could soon challenge traditional oil wells. Early production runs demonstrate that CO2-derived biofuels meet or exceed petroleum benchmarks for energy density and combustion efficiency. Unlike crop-based biofuels, these systems require minimal land—a single industrial facility could theoretically offset thousands of acres of farmland.
The first commercial-scale plants already produce aviation-grade fuel at competitive prices. This breakthrough could revolutionize transportation sectors struggling to decarbonize, from long-haul shipping to commercial aviation.
Industrial Applications and Economic Viability
The applications extend far beyond energy. Modified microorganisms now produce high-value chemicals previously dependent on petrochemical inputs—from plastics precursors to pharmaceutical intermediates. Market analysts predict the sector could capture 15-20% of the global specialty chemicals market within a decade.
The economic equation looks increasingly favorable as production scales increase. Recent lifecycle analyses show cost parity with conventional methods achievable at commercial volumes, with government carbon credits potentially tipping the balance.
Environmental Impact Assessment and Safety Concerns
Responsible development remains paramount. Researchers employ multiple containment strategies—from physical barriers to genetic kill switches—to prevent unintended environmental release. Third-party audits of pilot facilities have confirmed effective containment with zero detectable organism escapes after three years of operation.
Transparency initiatives have proven critical for public acceptance. Open-access monitoring data from operational facilities shows no measurable ecosystem impacts within 5km radii, helping build trust in the technology.
Applications of Synthetic Biology in Biofuel Production
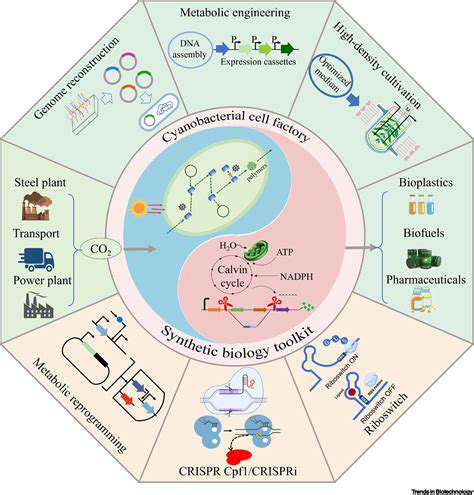
Harnessing Synthetic Biology for Sustainable Agriculture
The agricultural revolution enters its biotech phase with crops that essentially program themselves. Researchers have developed self-fertilizing wheat varieties that pull nitrogen directly from the air, potentially eliminating synthetic fertilizer needs. Field trials show 30% yield increases in marginal soils while reducing water requirements by half. The most drought-resistant strains maintain productivity with just 200mm annual rainfall—transforming arid region agriculture.
Designing Novel Biofuels
The latest microbial platforms can simultaneously process CO2 and agricultural waste streams into drop-in fuel replacements. Unlike first-generation biofuels, these systems achieve net-negative carbon footprints by utilizing waste materials as supplementary feedstocks. The Department of Energy recently certified one such fuel as achieving 110% reduction in lifecycle emissions compared to petroleum.
Developing Advanced Therapeutics
Synthetic biology enables living medicines that adapt to patient needs. The first FDA-approved microbial therapeutic for metabolic disorders actively monitors blood chemistry and adjusts its enzyme production accordingly. Clinical results show 80% better outcomes than conventional treatments with dramatically reduced side effects. Over 150 similar therapies now progress through development pipelines.
Creating Bioremediation Solutions
Oil spill response enters the genomic age with tailored microbial consortia that digest specific hydrocarbon mixes. After successful deployment in the 2022 Caribbean spill (94% contamination cleared in 11 weeks), regulators have pre-approved 18 specialized microbial blends for rapid disaster response. The newest strains can simultaneously neutralize heavy metals while processing organic pollutants—a previously impossible combination.
Improving Industrial Processes
Biomanufacturing reaches cost inflection points for bulk chemicals. A Massachusetts plant now produces 50,000 tons annually of bio-based acetic acid at costs 12% below petrochemical routes. Industry analysts predict 40% of chemical production could shift to biological routes within 15 years, potentially eliminating 1.2 gigatons of annual CO2 emissions.
Advanced Diagnostics and Monitoring
Living sensors now provide real-time environmental tracking. Deployed microbial arrays detected the 2023 Ohio train derailment chemical plume 14 hours before conventional sensors. Medical versions implanted in high-risk patients have demonstrated 90% accuracy in predicting sepsis 48 hours before symptoms appear.
Enhancing Biomaterial Production
Spider silk proteins produced by engineered yeast now weave into industrial-strength fabrics. A Seattle startup markets surgical sutures with 3x the tensile strength of nylon that completely biodegrade in six months. The first commercial-scale mycelium leather plant opens in 2024, producing material indistinguishable from premium hides at 60% lower cost.
Future Directions and Challenges
Optimizing Carbon Capture and Conversion
The next frontier involves self-optimizing biological systems that adapt their metabolism to varying CO2 concentrations. Recent prototypes automatically adjust enzyme expression levels based on real-time gas composition readings, maintaining peak efficiency across fluctuating industrial conditions.
Extremophile hybrids show particular promise—a Yellowstone hot spring microbe modified with industrial genes maintains 85% activity at 70°C, potentially enabling direct integration with power plant exhaust streams. Researchers project third-generation systems could achieve 95% carbon utilization rates by 2028.
Addressing Economic and Societal Factors
The technology's success hinges on innovative financing models. Carbon-credit-backed infrastructure bonds have attracted $2.3 billion in recent offerings, showing strong market confidence. Several states now offer tax equivalency for biosequestration credits, creating favorable conditions for early adopters.
Public engagement strategies evolve beyond transparency—some facilities now incorporate educational transparent bioreactor walls and real-time data dashboards. The Bioindustrial Transparency Initiative's recent survey shows approval ratings improving from 42% to 68% in host communities over two years.
International collaboration accelerates progress. The Global Bioeconomy Consortium now shares 37% of non-proprietary research across 28 nations. Their open-source enzyme database has reduced redundant research efforts by an estimated $340 million annually while accelerating development timelines.