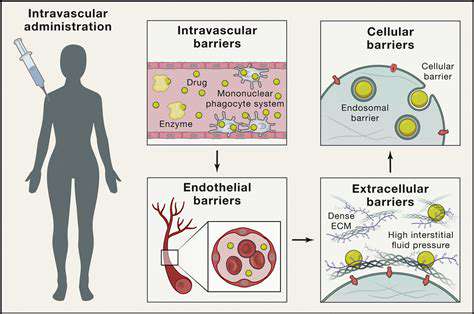
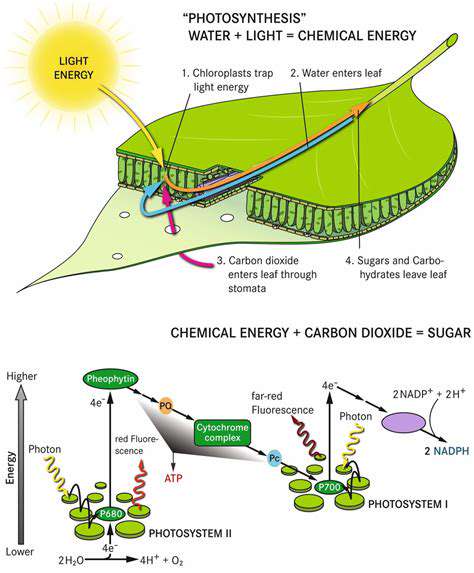
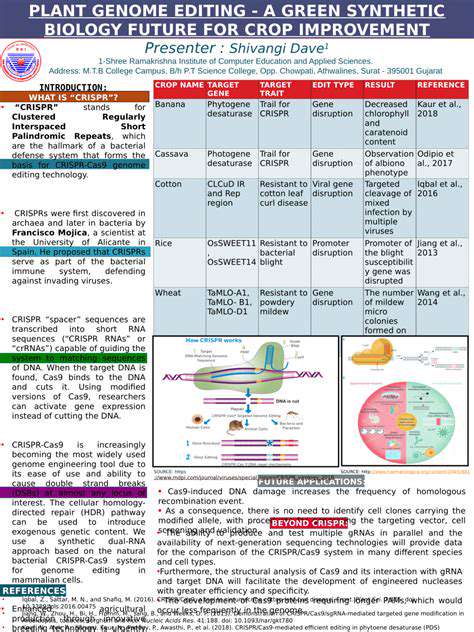
The CRISPR-Cas system, originally discovered as part of bacterial immune defenses, represents a groundbreaking method for addressing viral infections. This technology enables precise identification and modification of specific viral genetic sequences, effectively stopping viral reproduction and reducing infection potential. The exceptional accuracy of these systems makes them particularly valuable for creating new antiviral treatments, potentially transforming our approach to everything from seasonal illnesses to global health crises. Different CRISPR variants allow customization for targeting various viral threats.
At its core, the process involves a specialized RNA molecule that directs Cas enzymes to specific viral genetic material. Upon locating the target, these enzymes can either sever the viral DNA/RNA strands to halt replication or suppress viral gene activity, preventing production of essential viral components. This precision targeting spares healthy host cells, offering a significant improvement over conventional antivirals that often affect non-infected tissues.
Specific Viral Pathogen Applications
Current research explores CRISPR applications against numerous viruses including HIV, influenza strains, and coronaviruses, presenting novel therapeutic possibilities. Potential uses range from blocking viral entry into cells to disrupting essential viral functions. This technology could potentially address active infections, prevent new ones, and eliminate dormant viral reservoirs, representing a fundamental shift in antiviral treatment paradigms.
Beyond treatment, CRISPR systems show promise for preventive medicine. Genetic modifications could potentially enhance cellular resistance to viral invasion, paving the way for innovative vaccine approaches. Diagnostic applications are equally promising, with CRISPR-based tests enabling rapid, accurate viral detection for timely intervention and outbreak control. These developments mark significant progress in viral disease management.
The precision targeting capability of CRISPR technology offers tremendous potential for creating customized treatments against diverse viral threats, potentially transforming global health outcomes. Continued investigation remains essential to fully realize this technology's antiviral potential.
Specific Mechanisms of CRISPR-Cas Action Against Viruses
Targeting Viral Genetic Material
CRISPR-Cas systems employ multiple strategies to identify and modify viral DNA or RNA. This selective targeting forms the basis for effective antiviral defense while preserving host cell integrity. The system's specificity helps minimize unintended genetic alterations, a crucial factor for therapeutic safety.
Initial viral genetic identification typically involves unique viral sequences. The CRISPR RNA (crRNA) component, when combined with Cas proteins, ensures accurate target recognition through complementary base pairing.
Cas Protein Functional Diversity
Different Cas proteins perform specialized roles within the CRISPR system. Some specialize in genetic material cleavage, while others participate in initial viral detection. Understanding these varied functions is essential for developing effective antiviral strategies.
The functional diversity among Cas proteins demonstrates the system's adaptability against different viral genetic structures, supporting its potential for broad antiviral applications.
Disrupting Viral Reproduction
Beyond direct genetic modification, CRISPR can interfere with viral replication by targeting critical viral genes or regulatory elements. This indirect approach can effectively halt viral spread without complete genome destruction, offering valuable alternatives for infection control.
Targeting Precision
The accuracy of CRISPR targeting is paramount for therapeutic safety. Minimizing off-target effects remains a critical focus for developing safe antiviral applications. Ongoing research continues to refine targeting specificity, reducing risks to host cells while maintaining antiviral effectiveness.
crRNA's Critical Role
The crRNA molecule serves as the targeting guide for Cas proteins, ensuring precise viral sequence recognition through complementary binding. Understanding crRNA function is essential for optimizing CRISPR's antiviral potential.
Delivery System Challenges
Effective delivery of CRISPR components to infected tissues presents significant challenges for clinical applications. Current research focuses on developing reliable delivery methods and targeted approaches to viral reservoirs within the body.
Current Limitations and Research Directions
While promising, CRISPR antiviral applications face challenges including delivery optimization and potential viral resistance development. Future research must address these limitations while exploring new approaches to enhance system efficacy and safety.
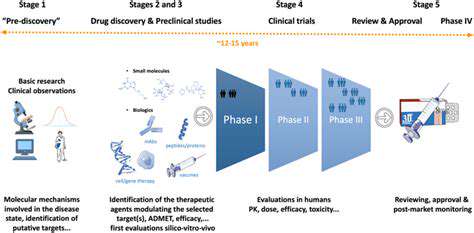
Clinical Applications and Future Directions

Advanced Diagnostic Applications
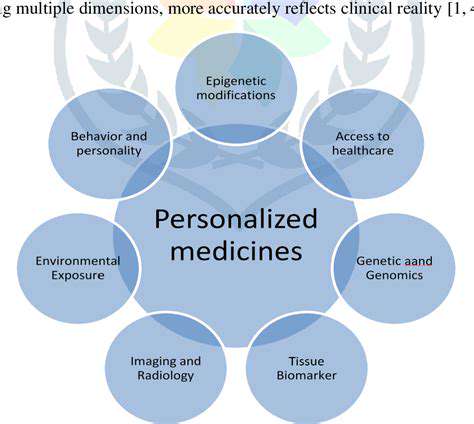
Modern diagnostic technologies are transforming medical practice by enabling earlier, more precise disease identification. These advancements are particularly valuable for personalized medicine, facilitating customized treatment plans based on individual patient profiles. Early disease detection, especially for conditions like cancer, can significantly improve treatment success rates and patient survival, leading to better overall healthcare outcomes.
Imaging Technology Advancements
The future of medical imaging looks particularly promising. AI-enhanced image analysis promises to accelerate and improve diagnostic accuracy, enabling faster identification of abnormalities and treatment initiation. Combining advanced imaging with other diagnostic methods will provide more comprehensive disease understanding, supporting more holistic patient care approaches.
Treatment Strategy Impacts
Modern diagnostics significantly influence treatment planning. By providing detailed disease information, these tools help clinicians select optimal, personalized treatments. This tailored approach improves patient outcomes while reducing risks from inappropriate therapies, representing a major advancement in medical practice.
Molecular Diagnostic Progress
Molecular diagnostics continue to advance, revealing genetic and molecular disease mechanisms. These developments enable detection of disease-associated genetic variations, supporting earlier, more accurate diagnoses. This precision medicine approach can dramatically alter treatment strategies, optimizing outcomes while reducing unnecessary treatments, representing a fundamental shift toward individualized care.
Health Record Integration
Seamless integration of diagnostic results with electronic health records improves healthcare efficiency. Centralized access to comprehensive patient data enhances care coordination, supporting more effective patient management through integrated information systems.
Ethical Considerations
The expansion of advanced diagnostics raises important ethical questions regarding data security and equitable access. Ensuring fair access to these technologies is essential to prevent worsening health disparities, requiring careful consideration during implementation.
Accessibility Challenges
The economic feasibility of advanced diagnostics significantly impacts their widespread adoption. Developing strategies to improve accessibility across diverse populations is crucial for maximizing benefits, with potential long-term improvements in health outcomes justifying necessary investments.
Ethical Implications and Considerations
Gene Editing Ethics
While CRISPR offers potential viral infection cures, it raises significant ethical concerns regarding genome modification. The technology's power necessitates careful consideration of potential misuse for non-therapeutic enhancements. We must establish robust ethical frameworks to ensure responsible application while preventing harmful uses.
Equitable access represents another critical concern. If CRISPR therapies become prohibitively expensive, they could worsen existing healthcare inequalities. Ensuring fair access is essential to prevent genetic medicine from becoming a privilege of wealth.
Germline Modification Complexities
Germline editing presents unique ethical challenges as modifications affect future generations. The potential for unforeseen, long-term population impacts requires extreme caution and thorough research before considering such interventions. Societal and evolutionary consequences demand careful public discussion and consensus-building.
Informed Decision-Making
Patients considering CRISPR treatments require comprehensive information about potential benefits, risks, and alternatives. True informed consent depends on transparent communication about both short- and long-term implications, ensuring autonomous healthcare decisions free from coercion.
Access Equity
CRISPR therapy development must prioritize equitable global access. Addressing socioeconomic, geographic, and cultural barriers is essential to prevent this technology from benefiting only privileged populations, requiring intentional design of distribution systems.
Unintended Effects
While highly specific, CRISPR carries some risk of unintended genetic modifications. Rigorous testing and long-term monitoring systems are essential to minimize risks and ensure therapeutic benefits outweigh potential harms, requiring ongoing safety evaluation frameworks.
Societal Considerations
CRISPR's introduction requires broad societal engagement to address concerns and establish consensus. Inclusive dialogue among scientists, ethicists, policymakers, and the public is essential for navigating this technology's complex ethical landscape and ensuring beneficial applications for humanity.