Introduction to Modern Oncology Drug Development
Advancements in Target Identification and Validation
Modern oncology drug development begins with the identification of specific molecular targets associated with cancer progression. Researchers utilize advanced genomic and proteomic technologies to uncover novel targets that are critical for tumor growth and survival. Validation of these targets involves rigorous laboratory experiments and clinical data analysis to ensure their relevance and potential for therapeutic intervention.
With the rise of personalized medicine, understanding the genetic and molecular landscape of individual tumors allows for more precise target selection. This approach increases the likelihood of developing effective drugs with fewer off-target effects, ultimately improving patient outcomes. The integration of bioinformatics and high-throughput screening plays a crucial role in accelerating this phase of drug discovery.
Innovations in Drug Design and Screening Technologies
Once targets are validated, medicinal chemists employ innovative drug design techniques such as structure-based drug design and computer-aided modeling to create potent compounds. These methods enable the optimization of drug candidates for better efficacy, stability, and safety profiles. High-throughput screening technologies facilitate rapid testing of thousands of compounds to identify promising leads efficiently.
Artificial intelligence and machine learning are increasingly integrated into this stage, allowing for predictive modeling of drug-target interactions. This technological synergy reduces development time and costs while increasing the precision of candidate selection, ultimately leading to more effective oncology therapies.
Development of Targeted Therapies and Biologics
Targeted therapies, including small molecules and monoclonal antibodies, have revolutionized cancer treatment by specifically attacking cancer cells while sparing healthy tissue. Developing these biologics requires a deep understanding of tumor-specific antigens and signaling pathways. Advances in recombinant DNA technology enable the production of highly specific antibodies that can block tumor growth or mark cancer cells for immune destruction.
Moreover, the development of antibody-drug conjugates combines the targeting precision of antibodies with the potency of cytotoxic drugs. These complex biologics are designed to deliver drugs directly to cancer cells, minimizing systemic toxicity and enhancing treatment efficacy.
Overcoming Resistance and Combining Therapies
Cancer cells often develop resistance to single-agent therapies, posing a significant challenge in oncology. Modern research focuses on understanding the mechanisms of resistance, such as genetic mutations or pathway redundancies, to develop strategies that can overcome them. Combination therapies, utilizing multiple drugs targeting different pathways, are a common approach to prevent or delay resistance.
Developing effective combination regimens requires careful assessment of drug interactions and synergistic effects. Innovative clinical trial designs, including adaptive trials, facilitate the rapid evaluation of these combinations, offering new hope for resistant cancers.
Role of Biomarkers in Precision Oncology
Biomarkers are essential for identifying patients who are most likely to benefit from specific therapies. They help in diagnosing, predicting treatment response, and monitoring disease progression. The discovery of predictive biomarkers enables clinicians to personalize treatment plans, improving efficacy and reducing unnecessary exposure to ineffective drugs.
Advances in liquid biopsy technologies, such as circulating tumor DNA analysis, provide minimally invasive means to track tumor evolution and resistance mechanisms in real time. This dynamic approach to biomarker assessment is transforming the landscape of precision oncology.
Regulatory Pathways and Accelerated Approval Processes
The regulatory landscape for oncology drugs has evolved to accommodate the urgent need for new therapies. Agencies like the FDA and EMA have implemented accelerated approval pathways, such as breakthrough therapy designations, to facilitate faster access to promising treatments for patients with unmet needs. These pathways often rely on surrogate endpoints and adaptive trial designs to expedite approval.
While accelerated approval can bring effective drugs to market more quickly, it also requires rigorous post-marketing studies to confirm clinical benefit. Balancing rapid access with safety and efficacy remains a critical aspect of modern oncology drug development.
High-Throughput Screening and Computational Drug Design
Introduction to High-Throughput Screening in Oncology Drug Discovery
High-throughput screening (HTS) has revolutionized the process of identifying potential anticancer compounds by enabling rapid testing of thousands to millions of chemical entities against specific biological targets. This technology accelerates the discovery pipeline, drastically reducing the time required to find promising candidates for further development. In oncology, HTS allows researchers to efficiently evaluate vast compound libraries for activity against cancer-related proteins, pathways, or cellular models, providing a crucial starting point for drug development.
By integrating automation, miniaturization, and advanced detection methods, HTS facilitates the pinpointing of lead compounds with desirable biological activity. This approach not only enhances the efficiency of initial screening but also helps uncover novel mechanisms of action, which can be vital in tackling drug-resistant cancer types. As a result, HTS remains an indispensable tool in modern oncology drug discovery efforts, paving the way for targeted therapies and personalized medicine.
Role of Computational Drug Design in Oncology
Computational drug design employs advanced algorithms, molecular modeling, and simulations to predict how potential drug molecules interact with cancer targets. This method complements traditional laboratory techniques by providing insights into binding affinities, conformations, and the dynamics of drug-target interactions, thereby streamlining the discovery process. In oncology, computational approaches help identify novel binding sites and optimize lead compounds for increased efficacy and reduced toxicity.
By leveraging vast amounts of biological data, computational drug design can prioritize the most promising candidates before synthesis and testing, saving considerable time and resources. Techniques such as structure-based drug design, virtual screening, and quantitative structure-activity relationships (QSAR) are extensively used to develop tailored therapies for specific cancer subtypes. Overall, computational methods are transforming oncology drug discovery into a more precise and predictive science.
Synergy Between High-Throughput Screening and Computational Methods
The integration of HTS with computational drug design creates a powerful synergy that enhances the efficiency and success rate of oncology drug discovery. Data generated from HTS can be fed into computational models to refine hypotheses, identify structure-activity relationships, and predict off-target effects. Conversely, computational predictions can guide HTS campaigns by narrowing down the vast chemical space to the most promising candidates for testing.
This iterative cycle accelerates the identification of lead compounds with optimal properties, reduces costs, and minimizes late-stage failures. In oncology, such combined approaches enable researchers to rapidly respond to emerging cancer targets and resistance mechanisms, ultimately leading to more effective and personalized treatments for patients.
Challenges and Limitations of High-Throughput Screening in Oncology
Despite its advantages, high-throughput screening in oncology faces several challenges, including the complexity of cancer biology and the limitations of in vitro models. Many compounds show promising activity in cell-based assays but fail in clinical settings due to poor bioavailability, toxicity, or off-target effects. Additionally, screening assays may not fully recapitulate the tumor microenvironment, leading to false positives or negatives.
Another limitation is the enormous data management and analysis required to interpret HTS results effectively. The potential for high false discovery rates necessitates rigorous validation and secondary screening. Addressing these challenges requires integrating HTS with other techniques, such as computational modeling and advanced imaging, to improve predictive accuracy and clinical relevance in oncology drug discovery.
Advances in Technology Enhancing HTS and Computational Design
Recent technological advancements have significantly improved the capabilities of high-throughput screening and computational drug design. Innovations such as next-generation sequencing, machine learning, and artificial intelligence (AI) enable more precise target identification and data analysis. These tools facilitate the handling of complex datasets and improve the prediction of compound efficacy and safety profiles.
Furthermore, developments in microfluidics and automation have increased screening throughput and reduced costs, making these technologies more accessible. Enhanced computational power allows for more accurate simulations of drug-target interactions and the exploration of vast chemical spaces. Collectively, these innovations are driving the future of oncology drug discovery toward faster, more accurate, and personalized therapeutic development.
Future Perspectives and Integrative Approaches
The future of oncology drug discovery lies in the seamless integration of high-throughput screening, computational modeling, and other emerging technologies such as genomics and proteomics. Such multidisciplinary approaches promise to uncover novel targets and develop highly selective therapies tailored to individual patient profiles. Advances in personalized medicine will depend heavily on these synergistic methods to identify effective treatments with minimal adverse effects.
As the field evolves, the emphasis will shift toward creating more predictive models that incorporate tumor heterogeneity, immune responses, and resistance mechanisms. The ongoing development of AI-driven algorithms and high-content screening platforms will further enhance the precision and speed of drug discovery. Ultimately, these integrated strategies aim to deliver more effective, safer, and personalized oncology treatments in the coming decades.
Overcoming Challenges in Oncology Drug Development
Understanding the Complexity of Cancer Biology
Cancer is an incredibly Heterogeneous disease, characterized by diverse genetic mutations and cellular pathways that vary significantly among patients. This complexity makes it difficult for researchers to identify universal targets for therapy, requiring a deep understanding of tumor biology at both molecular and cellular levels. Advances in genomics and proteomics have been instrumental in revealing the intricate networks that drive tumor growth and resistance, thus guiding the development of more precise therapeutic interventions.
Moreover, the dynamic nature of tumor evolution poses additional challenges, as cancer cells can adapt rapidly to therapeutic pressures, leading to drug resistance. Researchers must consider not only the static features of tumors but also their capacity to change over time. This necessitates continuous monitoring and adaptive treatment strategies, which are critical components in overcoming the biological hurdles inherent in oncology drug development.
Addressing Drug Resistance in Oncology
One of the most significant obstacles in developing effective cancer therapies is the emergence of drug resistance, which can occur through various mechanisms such as genetic mutations, activation of alternative signaling pathways, or phenotypic changes like epithelial-mesenchymal transition. Overcoming resistance requires a multifaceted approach, including combination therapies that target multiple pathways simultaneously to prevent tumor escape.
Innovative strategies such as personalized medicine, where treatments are tailored based on the genetic profile of individual tumors, are proving promising. Combining targeted agents with immunotherapies or chemotherapy can also enhance efficacy and delay resistance. Continuous research into resistance mechanisms is vital for designing next-generation drugs that can sustain long-term responses in patients.
Innovations in Biomarker Development
Biomarkers play a crucial role in predicting treatment responses and monitoring disease progression. Developing reliable, sensitive, and specific biomarkers remains a major challenge, yet it is essential for enabling precision oncology. Advances in liquid biopsies, which analyze circulating tumor DNA, offer minimally invasive options for real-time tumor profiling and early detection of resistance.
Furthermore, integrating multi-omics data—combining genomics, transcriptomics, proteomics, and metabolomics—can help identify novel biomarkers that reflect tumor biology more comprehensively. These innovations facilitate better patient stratification and enable clinicians to select the most effective therapies, ultimately improving clinical outcomes.
Overcoming Regulatory and Safety Challenges
Regulatory pathways for oncology drugs are often complex, given the need to balance expedited access to promising therapies with thorough safety evaluations. Navigating this landscape requires close collaboration between researchers, pharmaceutical companies, and regulatory agencies to establish clear guidelines that facilitate timely approval without compromising safety.
Safety concerns, particularly with combination therapies or novel agents, demand rigorous preclinical testing and vigilant clinical monitoring. Developing standardized protocols for adverse event reporting and patient safety assessments is essential for advancing innovative treatments while safeguarding patient well-being.
Enhancing Clinical Trial Design and Patient Recruitment
Designing effective clinical trials in oncology involves addressing challenges such as patient heterogeneity, recruitment difficulties, and trial costs. Adaptive trial designs, which allow modifications based on interim results, are increasingly used to improve efficiency and accelerate drug development. These designs enable faster evaluation of promising therapies and reduce resource expenditure.
Recruitment can be improved through better patient engagement, education, and the use of digital platforms to reach diverse populations. Incorporating real-world data and biomarkers into trial protocols also helps identify suitable candidates more effectively, ensuring that treatments are tested in appropriate patient subsets and increasing the likelihood of success.
Integrating Immunotherapy into Drug Development
Immunotherapy has revolutionized oncology, offering durable responses for some patients. However, integrating these therapies into drug development presents unique challenges, such as identifying predictive biomarkers for response and managing immune-related adverse events. Researchers are actively exploring combination strategies that include immunotherapies with targeted agents, chemotherapy, or radiation.
Developing effective immunotherapies requires a nuanced understanding of tumor immune evasion mechanisms and the tumor microenvironment. Advances in checkpoint inhibitors, CAR T-cell therapies, and cancer vaccines are expanding the arsenal against various cancers, but optimizing their use remains a key challenge for drug developers.
Fostering Collaboration and Data Sharing
Addressing the multifaceted challenges of oncology drug development necessitates collaboration across academia, industry, and healthcare systems. Sharing data, resources, and expertise accelerates discovery and reduces redundancy, ultimately benefiting patients. Initiatives such as public-private partnerships and consortia facilitate this exchange and promote standardization.
Open data sharing platforms enable researchers worldwide to analyze large datasets, identify new targets, and validate findings more rapidly. Such collaborative efforts are essential for overcoming the complexity of cancer and translating scientific discoveries into effective therapies that can improve patient outcomes on a global scale.
The Future of Drug Discovery in Oncology
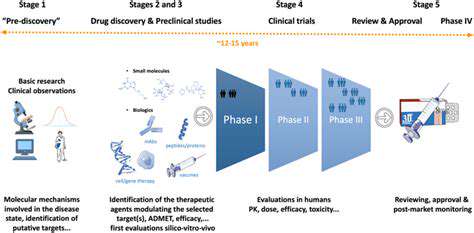
Accelerated Drug Discovery Through AI
Artificial intelligence (AI) is revolutionizing drug discovery, offering unprecedented speed and efficiency in identifying potential drug candidates. AI algorithms can analyze massive datasets of biological information, including genomic sequences, protein structures, and clinical trial data, to identify patterns and relationships that might be missed by human researchers. This accelerates the process of identifying promising drug targets and designing molecules with enhanced efficacy and reduced side effects. By automating many of the tedious and time-consuming tasks associated with traditional drug discovery, AI can significantly reduce the overall time and cost of bringing new treatments to market. The ability to quickly screen vast libraries of compounds allows researchers to focus on the most promising options, maximizing the chances of success.
Machine learning, a subset of AI, plays a crucial role in this transformation. Machine learning algorithms can learn from existing data to predict the properties of new compounds, such as their binding affinity to target proteins and potential toxicity. This predictive capability allows researchers to prioritize compounds with the highest likelihood of success, leading to more focused and efficient research efforts. The potential for Accelerated Drug Discovery through AI promises to drastically change the landscape of healthcare, leading to the development of more effective and personalized treatments for a wide range of diseases.
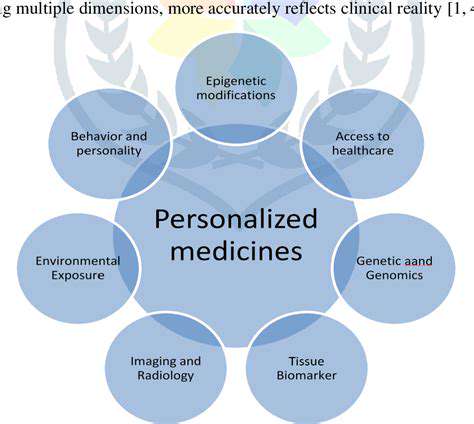
Personalized Medicine and Targeted Therapies
The future of drug discovery is increasingly focused on personalized medicine, tailoring treatments to individual patients based on their unique genetic makeup and other biological factors. This approach recognizes that diseases can manifest differently in different individuals, making it essential to develop therapies that target specific molecular pathways and genetic variations. This personalized approach can lead to more effective treatments with fewer side effects, improving patient outcomes and reducing healthcare costs.
Advances in genomics and proteomics are providing valuable insights into the individual variations that influence disease susceptibility and treatment response. By incorporating these insights into drug discovery processes, researchers can identify specific genetic markers that predict a patient's response to a particular drug. This targeted approach allows for the selection of treatments most likely to be effective and safe for each individual patient. This approach also allows for the development of new diagnostic tools to identify patients who would benefit most from specific treatments.
This approach is particularly important in treating complex diseases like cancer, where genetic mutations play a significant role in tumor development and progression. Personalized medicine can help in identifying the specific genetic alterations in a patient's tumor and selecting targeted therapies that effectively attack those specific mutations.