Imagine a world where genetic diseases could be edited out of existence. That's the promise of CRISPR-Cas9, a tool that's rewriting the rules of biotechnology and medicine. Unlike earlier gene-editing methods that often missed their mark, this system acts like molecular scissors with GPS-level accuracy.
What makes CRISPR-Cas9 extraordinary is its origin - it's borrowed from bacteria's ancient immune system, refined to perform surgeries on DNA strands. Researchers have observed its precision reduces collateral damage to surrounding genetic material, making it safer than previous approaches. The implications of this precision are only beginning to be understood.
Applications in Human Health
Clinics worldwide are reporting breakthrough cases where CRISPR therapies have successfully treated blood disorders like sickle cell anemia. The approach isn't limited to single-gene conditions - teams are developing strategies for complex neurological disorders and even HIV treatment.
What excites medical professionals most is the potential to correct genetic errors at their source rather than managing symptoms. This paradigm shift has led to unprecedented collaboration between academic institutions and pharmaceutical companies, accelerating progress in the field.
Ethical Considerations and Challenges
As the technology advances, bioethicists warn we're entering uncharted territory. The ability to edit human embryos raises difficult questions about where to draw the line between therapy and enhancement. International summits continue to debate appropriate safeguards.
The most pressing ethical dilemma involves whether we should use this technology to eliminate genetic diseases when it could also be used for non-medical enhancements. Legal experts emphasize the need for global standards to prevent a patchwork of conflicting regulations that could hinder responsible development.
Advancements in Research and Development
Laboratories are reporting new CRISPR variants that reduce errors by 90% compared to early versions. Some teams are developing CRISPR 2.0 systems that can edit RNA instead of DNA, offering temporary modifications for certain conditions. These innovations suggest we're only scratching the surface of what's possible.
Future Prospects and Societal Impact
Beyond medicine, agricultural scientists are using CRISPR to develop drought-resistant crops that could help address food insecurity. Environmentalists explore applications for conservation, such as modifying invasive species. The technology's versatility suggests it will touch nearly every aspect of modern life within our lifetimes. Policy makers struggle to keep pace with these rapid developments.
Historians may one day view CRISPR as the pivotal discovery that launched the next great technological revolution, comparable to the invention of the microscope or the computer.
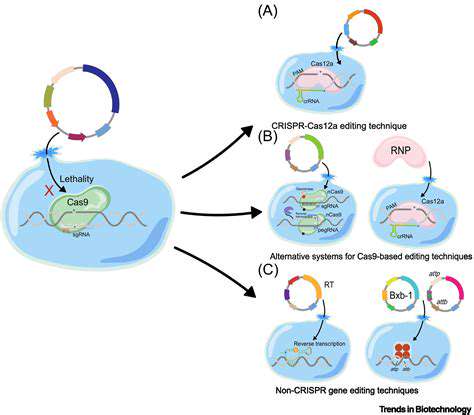
Precise Targeting for Disease Modification
Understanding the Need for Precise Targeting
Musculoskeletal disorders present unique challenges because they often involve multiple genetic factors working in concert. Traditional gene therapy approaches sometimes miss this complexity, like using a sledgehammer when a scalpel is needed. The latest research focuses on developing tools that can distinguish between nearly identical DNA sequences to avoid disrupting healthy genetic function.
Gene Editing Tools for Precise Targeting
While CRISPR remains the most well-known system, newer platforms like base editors offer intriguing alternatives. These function more like pencils than scissors, chemically rewriting individual DNA letters without cutting the strand. Early trials suggest they may cause fewer unintended changes while maintaining therapeutic effectiveness.
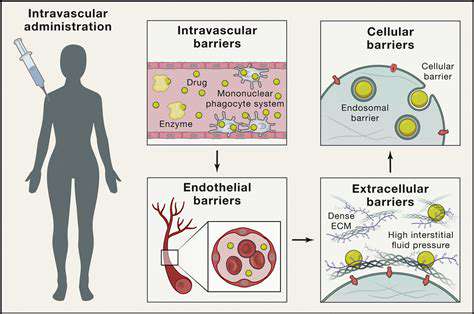
Delivery Systems for Efficient Gene Editing
The medical community is witnessing an explosion of novel delivery methods. Some labs report success with virus-like particles that can be programmed to seek out specific cell types. Others are experimenting with microscopic gene ferries that navigate the bloodstream to deposit their cargo precisely where needed.
Minimizing Off-Target Effects
Improved computational models now predict potential off-target sites with 95% accuracy before experiments begin. Some research groups have developed kill switches that automatically deactivate editing machinery after completing their task, adding an extra layer of safety.
Clinical Translation and Regulatory Considerations
Regulatory agencies have established rigorous pathways for gene therapy approval, requiring extensive preclinical data. The most promising approaches undergo multiple rounds of safety testing in animal models before human trials can commence. This cautious approach aims to balance innovation with patient protection.
Future Directions and Potential Applications
Emerging research explores combining gene editing with stem cell therapies to regenerate damaged cartilage and bone. Other teams are developing personalized approaches where a patient's own cells are edited outside the body then reintroduced. The next decade may see these technologies move from rare disease treatment to mainstream medical practice.