Viral Vectors: A Deep Dive
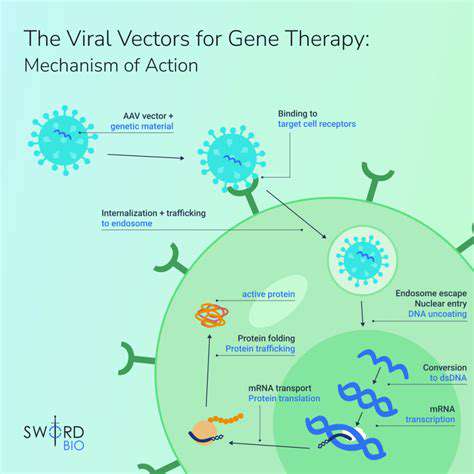
These engineered biological tools serve as indispensable vehicles in modern gene therapy applications. Modified viruses, stripped of their pathogenic properties yet retaining efficient gene delivery capabilities, enable targeted genetic interventions. The transformation from harmful pathogen to therapeutic ally requires precise genomic alterations that eliminate replication capacity while preserving transduction efficiency.
Genome editing precision stands as the cornerstone of viral vector safety and functionality. Researchers meticulously evaluate viral candidates based on their natural tropism and ability to penetrate specific cell types. This selective process ensures optimal delivery to intended cellular targets while minimizing off-target effects.
Types of Viral Vectors
The therapeutic toolbox includes several viral platforms, each offering distinct biological characteristics. Retroviral systems demonstrate unique genomic integration capabilities, potentially enabling permanent genetic modifications. Adenoviral platforms excel at transient expression scenarios where temporary genetic modulation suffices.
Adeno-associated viruses (AAVs) have gained prominence due to their favorable safety characteristics and sustained expression profiles. Their low immunogenicity and site-specific integration patterns make them particularly valuable for long-term therapeutic applications. Vector selection ultimately depends on the specific clinical requirements and desired duration of genetic effect.
Mechanism of Cellular Delivery
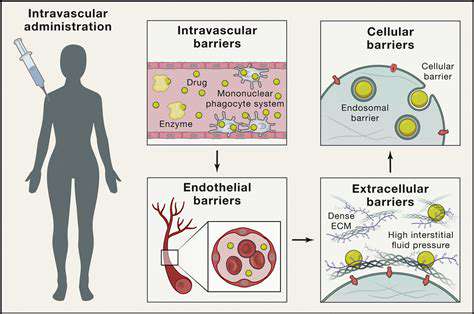
These biological delivery systems capitalize on evolutionary-refined viral entry mechanisms. Through sophisticated ligand-receptor interactions, they achieve efficient cellular penetration and payload delivery. The subsequent fate of the genetic material varies by vector type, ranging from episomal persistence to chromosomal integration.
Optimizing delivery protocols requires careful consideration of multiple variables. Administration route, vector dosage, and treatment timing collectively influence therapeutic outcomes. These parameters must be empirically determined for each clinical application through rigorous preclinical testing.
Safety and Performance Evaluation
Regulatory approval mandates comprehensive safety profiling of all viral therapeutics. Extensive characterization studies must verify the absence of residual pathogenicity and assess immunogenic potential. These evaluations include detailed analyses of vector purity, sterility, and genetic stability.
Potential genomic disruption represents a critical safety consideration, particularly for integrating vectors. Modern vector designs incorporate safeguards to minimize insertional mutagenesis risks through targeted integration strategies and self-inactivating configurations.
Therapeutic Applications
Genetic medicine has been transformed by these advanced delivery platforms, enabling treatment of previously intractable disorders. From monogenic diseases to complex malignancies, viral vectors facilitate targeted genetic interventions. Clinical successes have been documented across diverse conditions including hematologic, neurologic, and metabolic disorders.
The therapeutic landscape continues to expand rapidly as vector engineering advances overcome historical limitations. Current research focuses on enhancing delivery precision, modulating immune interactions, and developing regulatable expression systems to further improve clinical outcomes.
E-commerce platforms face significant operational challenges during peak traffic periods. Seasonal demand surges and viral marketing campaigns frequently reveal infrastructure vulnerabilities where content delivery networks provide distributed solutions.
Challenges and Future Directions

Technological Evolution
The relentless pace of innovation creates both opportunities and implementation challenges across industries. Maintaining technological competitiveness demands sustained R&D investment, presenting financial obstacles for resource-constrained organizations. This environment necessitates adaptive workforce development strategies to ensure continuous skill acquisition.
Data management represents another critical challenge in this digital era. The exponential growth of information requires sophisticated architectures for efficient processing, storage, and analysis to derive meaningful insights.
Economic Factors
Macroeconomic volatility significantly influences research funding and technology adoption rates. Financial constraints during economic contractions can delay critical innovation cycles and therapeutic advancements. Strategic financial planning and alternative funding models become essential for sustainable progress.
Implementation costs for cutting-edge technologies often create adoption barriers. Collaborative consortiums and public-private partnerships offer viable pathways to share development costs and accelerate innovation.
Social Impact
Technological disruption generates complex societal consequences that require proactive management. Workforce transformations and ethical considerations must be addressed through inclusive policy frameworks. Ensuring equitable technology access and benefit distribution remains fundamental to socially responsible innovation.
The digital divide presents ongoing challenges that demand targeted interventions. Inclusive design principles and accessibility considerations must be integrated throughout the development lifecycle.
Environmental Responsibility
Sustainable technology development has become an operational imperative across industries. Lifecycle analysis and circular economy principles must guide product development to minimize ecological impact. Energy-efficient computing architectures and renewable energy adoption represent critical focus areas.
Green technology initiatives now serve dual purposes - environmental protection and long-term economic viability. Sustainable practices have transitioned from optional enhancements to essential business requirements.
Clinical Trials and Emerging Therapies
Clinical Trial Methodologies
Neurological gene therapy trials demand exceptionally rigorous design parameters. Participant selection criteria must account for disease progression, genetic markers, and therapeutic window considerations. Comprehensive monitoring protocols should capture both immediate treatment effects and longitudinal outcomes.
Standardized assessment batteries ensure reliable data collection across study sites. Advanced statistical models must account for multiple variables while maintaining sufficient power to detect clinically meaningful effects. This methodological rigor remains essential for demonstrating therapeutic value and securing regulatory approval.
Innovative Treatment Approaches
Beyond conventional viral vectors, novel delivery systems are expanding therapeutic possibilities. Synthetic nanoparticles offer enhanced targeting capabilities and reduced immunogenicity compared to biological vectors. Genome editing platforms enable precise correction of pathogenic mutations at their source.
Cell-type specific targeting represents another promising frontier in neurological therapeutics. These approaches aim to maximize therapeutic impact while minimizing systemic exposure. The development of patient-specific therapies continues to advance, potentially offering customized solutions for genetically heterogeneous conditions.