The Revolutionary Nature of CRISPR-Cas
The CRISPR-Cas system, derived from bacterial immune systems, has fundamentally altered the landscape of genetic engineering. Its precision and programmability enable scientists to edit DNA with remarkable accuracy, surpassing older techniques that were often unpredictable. This groundbreaking approach provides an unparalleled method for gene manipulation, creating opportunities in diverse fields ranging from medical treatments to agricultural advancements.
Previous gene-editing tools frequently caused unintended changes and were inefficient, but CRISPR-Cas offers a more reliable and exact method. Such precision is essential in medical applications, where even minor errors could have serious consequences.
The Mechanism of CRISPR-Cas Action
CRISPR-Cas operates by employing a guide RNA molecule to locate a specific DNA sequence. Functioning like a molecular homing device, this RNA pinpoints the exact area of DNA that requires modification. The Cas enzyme, typically Cas9, then acts as molecular scissors, cleaving the DNA at the intended site.
This targeted cut enables researchers to either alter the DNA sequence or deactivate a particular gene. The cell’s natural repair mechanisms subsequently take over, facilitating the desired genetic change.
Applications in Gene Therapy
Among the most exciting uses of CRISPR-Cas technology is its potential in gene therapy. Scientists are investigating its ability to fix genetic mutations linked to diseases such as cystic fibrosis, sickle cell anemia, and Huntington’s disease. By accurately targeting and repairing defective genes, CRISPR-Cas could provide a lasting solution to these debilitating conditions.
Initial clinical trials are currently assessing the safety and effectiveness of CRISPR-Cas in treating various illnesses. While the possibilities are vast, ethical concerns and long-term impacts must be carefully evaluated.
CRISPR-Cas in Agricultural Biotechnology
Beyond healthcare, CRISPR-Cas is making significant strides in agricultural biotechnology. Researchers are leveraging it to create crops with improved nutritional content, greater resistance to pests and diseases, and higher yields. This could play a crucial role in addressing global food security and promoting sustainable farming practices.
By modifying crops to endure extreme conditions or enhance their nutrient profiles through targeted gene editing, scientists are unlocking new ways to meet the growing demands of food production.
Ethical Considerations and Societal Impact
The widespread use of CRISPR-Cas technology raises profound ethical questions. The ability to modify the human genome prompts concerns about potential misuse and the long-term implications of such interventions. Rigorous discussions and regulatory frameworks are necessary to ensure responsible development and application.
Engaging the public in meaningful dialogue and carefully weighing societal impacts are critical as the technology progresses, ensuring its benefits are distributed fairly and responsibly.
RNA-Guided Mechanisms in Other Systems
Although CRISPR-Cas is the most well-known, other RNA-guided gene-editing systems are emerging. Studying these alternatives could lead to new breakthroughs and expand the capabilities of genetic engineering. Exploring different approaches is vital for developing more versatile and effective tools for genome manipulation.
Investigating these alternative RNA-guided systems holds promise for uncovering novel mechanisms and applications that may surpass the limitations of CRISPR-Cas.
The Future of CRISPR-Cas Technology
CRISPR-Cas is continually evolving, with ongoing research focused on enhancing its accuracy, efficiency, and delivery methods. Future advancements are expected to unlock even more innovative applications, including sophisticated gene-editing tools and solutions for complex genetic disorders.
Efforts will likely concentrate on refining the system to overcome delivery challenges, improving its specificity, and extending its use to more intricate genetic conditions. This could herald a new era in genetic medicine and biotechnology.
Beyond CRISPR: Other RNA-Guided Nucleases
Beyond the CRISPR-Cas9 System: Exploring Alternative RNA-Guided Nucleases
While CRISPR-Cas9 dominates discussions on gene editing, numerous other RNA-guided nucleases exist, each with distinct features and applications. Understanding these alternatives is key to broadening the genetic engineering toolkit and addressing CRISPR’s limitations, such as off-target effects and delivery issues. This exploration underscores the vast potential beyond CRISPR-Cas9, paving the way for more precise and efficient genetic modifications.
The Role of Transcription Activator-Like Effector Nucleases (TALENs)
Transcription activator-like effector nucleases (TALENs) represent an earlier generation of RNA-guided nucleases. These engineered proteins target specific DNA sequences, creating double-strand breaks similar to CRISPR-Cas9. While TALENs have demonstrated efficacy in gene editing, their design and construction are more complex and time-consuming compared to CRISPR-Cas9.
Despite their initial prominence, TALENs have been largely overshadowed by CRISPR-Cas9 due to the latter’s simplicity. However, TALENs remain valuable for specific research applications where their unique properties offer advantages.
Zinc Finger Nucleases (ZFNs): A Pioneer in Gene Editing
Zinc finger nucleases (ZFNs) were among the first RNA-guided nucleases developed. They use engineered zinc finger proteins to bind to specific DNA sequences, inducing double-strand breaks for gene editing. Early research validated ZFNs’ potential, but their intricate design and off-target effects have led to reduced use in favor of CRISPR-Cas9.
Fn-Cas9: Expanding the CRISPR Toolbox
Fn-Cas9, a variant of the Cas9 protein, offers enhanced specificity and fewer off-target effects than traditional Cas9. This improvement is particularly valuable for therapeutic applications requiring high precision. Additionally, Fn-Cas9’s adaptability allows for targeting multiple genomic locations with greater accuracy.
Beyond the Double-Strand Break: Base Editing and Prime Editing
Traditional RNA-guided nucleases create double-strand breaks, but newer techniques like base editing and prime editing enable more precise modifications. These methods alter DNA bases directly without inducing breaks, reducing the risk of unintended mutations. Such advancements could transform gene therapy by providing safer and more controlled genetic corrections.
Investigating the Role of RNA-Guided Nucleases in Gene Regulation
Beyond editing, RNA-guided nucleases are being explored for gene regulation. By targeting specific DNA sequences, these tools can activate or suppress genes, offering novel therapeutic avenues. This precision in gene regulation represents a significant leap forward in treating genetic disorders.
Delivery Challenges and Future Directions
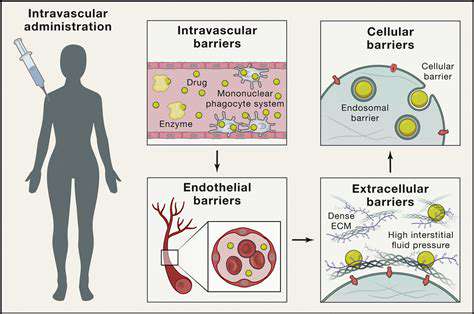
Delivering RNA-guided nucleases to target cells remains a major hurdle. Researchers are developing innovative delivery methods to improve efficiency and safety. Advances in this area are critical for translating these technologies into practical treatments. Continued progress will be essential to fully realize the potential of RNA-guided nucleases in medicine and beyond.
Applications in Gene Therapy and Biotechnology
RNA-Guided Nucleases in Gene Editing
RNA-guided nucleases, particularly CRISPR-Cas systems, have redefined gene editing. Their ability to target and modify DNA with precision has unlocked new possibilities in medicine and biotechnology. This capability allows researchers to correct mutations, introduce genes, and study gene function in unprecedented ways. The versatility of these tools is reshaping our approach to genetic research and therapy.
The underlying mechanisms are both intricate and elegant. Guide RNAs, tailored to match specific DNA sequences, direct Cas enzymes to the target site. The enzyme then creates a double-strand break, prompting cellular repair processes. These processes can be harnessed to introduce desired changes or disable genes, offering precise control over genetic material.
Applications in Gene Therapy
RNA-guided nucleases hold immense promise for gene therapy. By targeting disease-causing genes, they offer potential cures for previously untreatable conditions. For example, correcting mutations responsible for cystic fibrosis or sickle cell anemia could transform patients’ lives. Clinical trials are already underway, testing CRISPR-Cas systems for various inherited diseases. The goal is to develop safe and effective therapies that permanently address genetic defects.
Beyond inherited disorders, these technologies show potential in treating diseases like cancer. Targeting genes involved in cancer growth or suppression could lead to more precise and less toxic treatments. This precision opens new doors in oncology, offering hope for more effective therapies.
Applications in Biotechnology
The influence of RNA-guided nucleases extends to biotechnology, revolutionizing agriculture and industry. Crops can now be engineered for pest resistance, improved nutrition, and higher yields. Such advancements are critical for sustainable farming and global food security.
In industrial biotechnology, these tools are used to engineer microorganisms for producing biofuels, pharmaceuticals, and other valuable compounds. The precision of RNA-guided nucleases enables more efficient and environmentally friendly manufacturing processes. The potential applications continue to grow, driving innovation across multiple sectors.