Introduction to Drug Repurposing
Understanding Drug Repurposing
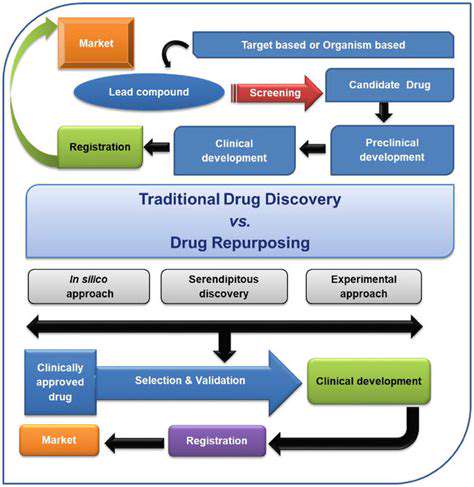
The concept of repurposing pharmaceuticals, often termed drug repositioning, represents a paradigm shift in therapeutic development. Rather than pursuing novel compounds, this methodology capitalizes on established medications with proven safety profiles to address alternative medical conditions. The approach harnesses decades of accumulated research data, bypassing many preliminary stages of traditional drug discovery.
This strategy can slash the typical decade-long development cycle by years, offering patients faster access to potentially life-saving treatments. By examining approved drugs through new therapeutic lenses, medical researchers can circumvent numerous regulatory hurdles while conserving billions in development costs that would otherwise be required for de novo drug creation.
The Importance of Drug Repurposing
Modern pharmacology faces mounting pressures from escalating R&D expenditures and diminishing returns on investment. Repositioning existing therapeutics emerges as a financially prudent solution, particularly for rare diseases and conditions lacking commercial incentives for traditional development. The approach proves especially valuable when rapid therapeutic responses are needed, such as during emerging pandemics.
Repurposed medications can provide immediate solutions for patients with limited treatment alternatives, particularly in specialty areas like neurodegenerative disorders or antibiotic-resistant infections where new drug development faces significant scientific barriers.
Identifying Potential Drug Candidates
The candidate selection process employs sophisticated computational biology techniques alongside traditional pharmacological assessments. Advanced bioinformatics platforms screen extensive compound libraries, analyzing molecular structures for potential off-target effects that might prove therapeutically beneficial. Machine learning algorithms now assist in predicting protein-drug interactions that human researchers might overlook.
This high-tech sifting process prioritizes compounds with optimal ADME (absorption, distribution, metabolism, excretion) profiles. Particular emphasis is placed on medications with extensive clinical histories, as their established safety data significantly reduces development risks compared to novel chemical entities.
Validation Through In Vitro and In Vivo Studies
Preclinical validation employs cutting-edge three-dimensional cell culture systems that better mimic human physiology than traditional monolayer cultures. These advanced models provide more accurate predictions of drug efficacy before progressing to animal testing. Researchers now utilize patient-derived xenograft models that incorporate actual human tumor tissues into immunocompromised rodents.
The transition to in vivo testing focuses on pharmacokinetic parameters and therapeutic windows. These comprehensive preclinical evaluations are vital for identifying potential toxicity issues before human exposure, particularly when considering drugs for pediatric or geriatric populations that may metabolize medications differently.
Clinical Trials and Regulatory Approvals
Human trials for repurposed drugs often benefit from accelerated pathways, particularly when targeting serious conditions with few alternatives. Adaptive trial designs allow for protocol modifications based on interim results, increasing efficiency. The FDA's 505(b)(2) pathway specifically facilitates approval of repurposed agents by permitting reliance on previously submitted safety data.
These streamlined regulatory processes can reduce clinical development timelines by 40-60% compared to novel drugs, while still maintaining rigorous safety standards. Post-marketing surveillance remains crucial for detecting rare adverse events that might emerge with broader usage patterns.
Advantages and Challenges of Drug Repurposing
The economic benefits are substantial - development costs typically range from $50-300 million versus $2-3 billion for new molecular entities. Patent extension strategies, including formulation patents and combination therapies, help maintain commercial viability. However, demonstrating convincing mechanistic rationale for new indications remains scientifically challenging.
The most successful repurposing cases often combine strong scientific rationale with clear unmet medical need, such as thalidomide's reinvention for multiple myeloma after its notorious history. Overcoming clinician skepticism about old drugs for new uses requires robust clinical evidence and education.
Future Directions and Applications
Next-generation repurposing integrates multi-omics approaches, combining genomic, proteomic and metabolomic data to identify novel drug-disease connections. Blockchain technology is being explored for secure sharing of repurposing data across institutions. Academic drug discovery consortia are forming to systematically evaluate off-patent medications for new applications.
Artificial intelligence systems now analyze millions of patient records to detect serendipitous treatment benefits, uncovering patterns invisible to human researchers. These technological advances promise to exponentially increase repurposing success rates across therapeutic areas.
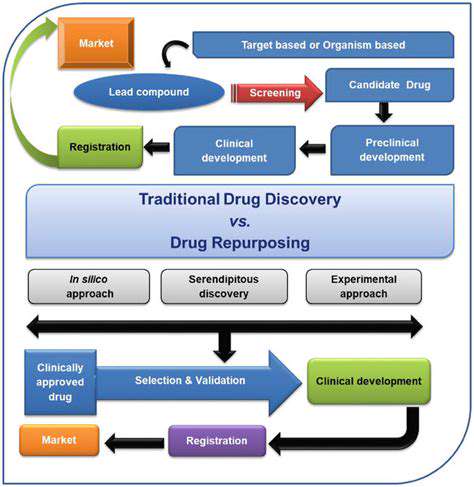
Challenges and Opportunities in Drug Repurposing
Identifying Suitable Candidates
The candidate selection process for neglected tropical diseases requires innovative approaches. Researchers are mining electronic health records from endemic regions for unexpected therapeutic correlations. Phenotypic screening in engineered parasite strains helps identify compounds with activity against multiple life cycle stages. Computational chemists are developing specialized algorithms to predict activity against eukaryotic pathogens based on mammalian target data.
Overcoming Regulatory Hurdles
Regulatory agencies have implemented special programs like the FDA's Tropical Disease Priority Review Voucher program to incentivize repurposing for neglected diseases. Novel trial designs incorporating real-world evidence are gaining acceptance. The European Medicines Agency's PRIME scheme offers enhanced support for repurposed medicines addressing unmet needs.
Addressing Practical and Ethical Considerations
Creative licensing models like the Medicines Patent Pool are enabling broader access to repurposed medicines. Differential pricing strategies help ensure affordability in low-income countries while maintaining commercial viability. Ethical frameworks now emphasize equitable benefit-sharing when repurposing traditional medicines with indigenous knowledge origins.