The Peculiar Challenges of Rare Disease Drug Development
Defining and Diagnosing Rare Diseases
Rare diseases, encompassing a vast spectrum of conditions affecting a small portion of the population, present significant hurdles in drug development. Precisely defining these conditions, often characterized by overlapping symptoms and complex genetic underpinnings, is a critical first step. Accurate diagnosis is frequently delayed, as symptoms can mimic those of more common ailments, leading to misdiagnosis and potentially inappropriate treatments. This diagnostic challenge directly impacts the ability to assemble robust clinical trial populations.
Limited Patient Populations
One of the most significant obstacles in rare disease drug development is the extremely small number of patients affected by a specific condition. This limited patient pool poses challenges in recruiting participants for clinical trials, which often require a large number of subjects to demonstrate a statistically significant treatment effect. Finding enough patients with a specific genetic mutation or symptom profile to constitute a meaningful dataset is an immense logistical and financial undertaking.
Varied Disease Presentations
Rare diseases exhibit a wide range of manifestations, both in terms of symptoms and severity. This variability often makes it difficult to standardize clinical trial protocols and outcomes measures. A drug that might be effective for one patient presenting with a specific subtype of a rare disease may not be effective for another patient with a different presentation of the same condition. Developing a trial design that captures this heterogeneity while still maintaining statistical rigor is a complex undertaking.
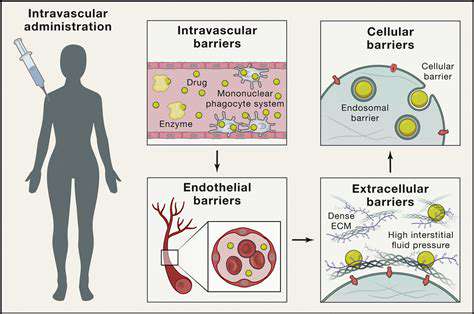
Developing Effective Animal Models
Reliable animal models are crucial for preclinical testing of potential therapies. However, creating animal models that accurately reflect the complexities of human rare diseases is often challenging. The genetic and environmental factors that contribute to the disease in humans are difficult to replicate in animal systems. Consequently, preclinical results may not always translate effectively to human clinical trials.
Funding and Resources
Drug development for rare diseases is often underfunded compared to research on more prevalent conditions. Limited financial resources can hinder the ability of researchers to conduct comprehensive preclinical studies, assemble large-scale clinical trials, and support the long-term follow-up required to assess the efficacy and safety of potential therapies. This financial constraint contributes significantly to the slow pace of development in this area.
Ethical Considerations in Research
Ethical considerations play a crucial role in rare disease drug development. Ensuring patient safety, obtaining informed consent from individuals with limited life expectancies, and balancing the potential benefits of a new therapy against the inherent risks are paramount. Ethical review boards must carefully evaluate the feasibility of trials in these populations, while also striving to include underrepresented patient groups in research.
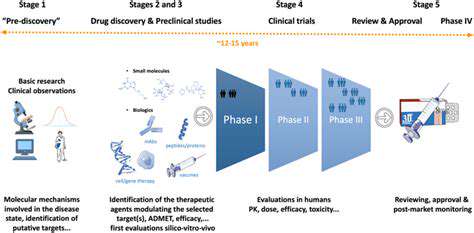
Clinical Trial Design and Regulatory Considerations

Ethical Considerations in Clinical Trial Design
Clinical trials, while crucial for advancing medical knowledge and improving patient care, must be conducted ethically. Protecting the rights and well-being of participants is paramount, and rigorous oversight is essential throughout the entire trial process. This includes obtaining informed consent, ensuring that participants understand the potential risks and benefits of the trial, and respecting their autonomy to withdraw at any time. Furthermore, protocols must address potential biases and ensure equitable access to the trial for diverse populations. Maintaining confidentiality and data security for participants is also a critical ethical concern. The use of placebos and the appropriate management of adverse events must be carefully considered and aligned with ethical guidelines.
Independent ethics committees (IECs) play a vital role in safeguarding the ethical conduct of clinical trials. These committees review trial protocols to assess their adherence to ethical principles. Their review process ensures that trials are designed and implemented in a manner that minimizes risks to participants and maximizes potential benefits. Ethical considerations are not simply a box to tick; they are integral to the entire research process, shaping the trial's design and implementation. The ethical dimensions of clinical trials extend beyond the specific study itself, impacting the broader scientific community and the trust placed in medical research.
Regulatory Frameworks for Clinical Trials
Clinical trials are subject to stringent regulatory oversight to ensure data integrity, participant safety, and the validity of research findings. These regulations are designed to protect both participants and the public by establishing standards for trial design, implementation, and data analysis. These regulations also aim to guarantee that the research is conducted in a transparent and accountable manner. Adherence to these regulations is vital for maintaining public trust in medical research and for ensuring that the results of clinical trials can be relied upon to inform clinical practice and public health policies.
Specific regulatory bodies, like the FDA in the United States, have established guidelines and regulations that govern the conduct of clinical trials. These guidelines cover various aspects, from the initial design and implementation to the reporting of results. Compliance with these rigorous regulations is crucial for ensuring the credibility and reliability of clinical trial findings, ultimately influencing how these findings are used to improve healthcare practices.
The regulatory landscape surrounding clinical trials is complex and constantly evolving. Researchers and trial sponsors must stay abreast of these changes to ensure their studies meet the highest standards of ethical and regulatory compliance. This continuous adaptation to regulatory changes is critical for maintaining the integrity of clinical trials and promoting the advancement of medical knowledge.
Thorough understanding and diligent adherence to regulatory guidelines are crucial for the successful completion of clinical trials.
Future Directions and Emerging Technologies
Artificial Intelligence Integration
Integrating AI into various aspects of the field presents exciting possibilities. AI-powered tools can automate repetitive tasks, freeing up human resources for more complex and creative endeavors. This automation could lead to significant improvements in efficiency and productivity, ultimately impacting the quality and speed of the work produced. AI can also be leveraged to analyze vast datasets, identifying patterns and insights that might otherwise remain hidden.
Furthermore, AI could personalize experiences for users, tailoring content and services to individual needs. This customization could enhance user engagement and satisfaction while also improving the overall effectiveness of the field.
Enhanced User Experience
A focus on user experience is crucial for continued success. This includes providing intuitive interfaces, seamless navigation, and engaging content. By prioritizing user experience, we can ensure that the field remains accessible and enjoyable for a wider audience, fostering broader adoption and participation. Accessibility features and diverse content formats are also important considerations.
User feedback mechanisms will be vital to understanding and addressing user needs. Gathering and analyzing this feedback will allow us to adapt and refine our approaches, continuously improving the overall user experience.
Data Security and Privacy
With increased reliance on data, robust data security and privacy measures are paramount. Protecting sensitive user information is essential to maintain trust and confidence. Implementing strong encryption protocols, access controls, and data anonymization techniques will be critical to safeguarding user data and adhering to privacy regulations.
Regular security audits and vulnerability assessments are necessary to proactively identify and mitigate potential risks. Transparency about data handling practices is also crucial to building trust and ensuring compliance.
Sustainability and Environmental Impact
Considering the environmental impact of our work is increasingly important. Sustainable practices, such as minimizing energy consumption and utilizing eco-friendly materials, should be prioritized in future developments. This conscious approach can significantly reduce the environmental footprint of the field and contribute to a more sustainable future.
Exploring alternative energy sources and optimizing resource utilization are crucial for environmentally responsible development. Measuring and reporting on environmental impact will be essential for tracking progress and identifying areas for improvement.
Ethical Considerations
As the field evolves, careful consideration of ethical implications is crucial. Addressing potential biases in algorithms and ensuring fairness and equity in outcomes is paramount. Robust ethical guidelines and frameworks should be developed and adhered to.
Promoting transparency and accountability in the development and deployment of new technologies is essential for responsible innovation. Engaging with diverse perspectives and stakeholders in the development process is vital to ensure ethical considerations are appropriately addressed.
Accessibility and Inclusivity
Ensuring accessibility and inclusivity for all users is fundamental to the success of the field. This includes designing for users with disabilities and diverse backgrounds. Providing multilingual support and accessibility features is crucial to fostering a more inclusive environment.
Addressing potential barriers to participation, such as cost and technical literacy, is essential. Educational resources and support programs can help bridge these gaps.
Emerging Technologies and Innovations
Staying abreast of emerging technologies and innovations is critical for maintaining a competitive edge. Exploring cutting-edge technologies like blockchain, quantum computing, and advanced materials will open up new possibilities and approaches. The incorporation of these technologies will lead to advancements in various aspects of the field.
Collaboration with researchers and innovators in other fields will be vital for exploring new frontiers and pushing the boundaries of what's possible. Open-source initiatives and collaborative platforms can foster innovation and knowledge sharing.