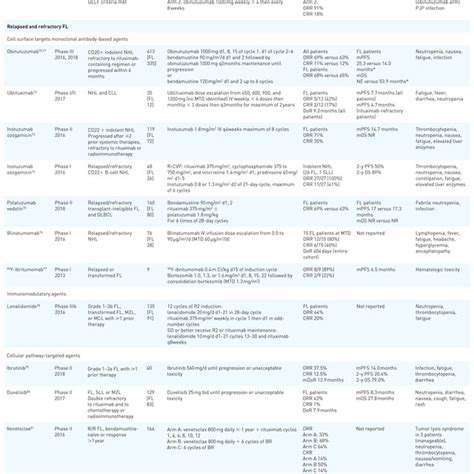
High-throughput screening (HTS) is a powerful approach in drug discovery and research, enabling scientists to rapidly evaluate a vast number of compounds or biological samples. This method significantly accelerates the process of identifying potential drug candidates, allowing for the exploration of a wider chemical space and accelerating the entire drug development pipeline.
By automating the testing process, HTS minimizes human error and maximizes efficiency. This automation is crucial for handling the large datasets generated during the screening process, ensuring reliable and consistent results.
Automation in HTS
Automation is fundamental to the efficiency of high-throughput screening. Sophisticated robotic systems handle sample preparation, compound dispensing, and data acquisition, freeing researchers from tedious manual tasks. This automation dramatically increases the throughput of experiments, allowing for the screening of thousands, or even millions, of compounds in a relatively short period.
Automated liquid handling systems, plate readers, and image analysis software work seamlessly together to process the large volume of data generated, allowing for rapid analysis and identification of promising hits. These automated systems are crucial for maintaining the accuracy and consistency needed for reliable results in large-scale screening.
Target Identification and Validation
A critical aspect of HTS is the identification and validation of target proteins or molecules. This process involves selecting specific targets believed to be involved in the disease process, and then designing assays to evaluate how compounds interact with these targets.
Validation ensures that the identified targets are indeed relevant to the disease and that the compounds effectively interact with these targets in a manner that could potentially lead to therapeutic outcomes. This crucial step is essential to avoid false positives and to focus research efforts on promising candidates.
Assay Development and Optimization
Developing and optimizing assays is a crucial step in HTS. Assays must be designed to be sensitive, specific, and high-throughput, enabling the accurate detection of interactions between compounds and target molecules. The accuracy and reliability of the data produced directly depend on the quality of the assays used.
Optimization of assays to achieve optimal performance is vital for accurate and reproducible results. This often involves iterative refinement of assay conditions, such as reagent concentrations, incubation times, and temperature, to maximize sensitivity and minimize background noise.
Data Analysis and Interpretation
The sheer volume of data generated in HTS requires sophisticated data analysis and interpretation techniques. Statistical methods are employed to identify significant hits and filter out false positives, ensuring that the most promising compounds are prioritized for further investigation.
Data visualization tools are crucial for identifying patterns and trends within the large datasets, allowing researchers to quickly identify compounds with desirable characteristics. Advanced algorithms and machine learning techniques are increasingly being used to analyze these complex datasets and identify potential drug candidates.
Hit Identification and Follow-up
Hit identification is a crucial step in HTS. Compounds that exhibit a desirable interaction with the target molecule in the initial screen are considered hits. These hits are then subjected to further validation and characterization to determine their potential as drug candidates.
Lead Optimization and Development
Lead optimization is the process of modifying identified hits to improve their potency, selectivity, and pharmacokinetic properties. This process aims to enhance the drug-like characteristics of the molecules and optimize their efficacy and safety. This step is a critical component of the drug discovery process and often involves iterative rounds of synthesis and testing.
The optimized compounds are then evaluated for their safety and efficacy in preclinical models, further refining their potential as therapeutic agents.
Robotic servicing technologies are paving the way for a new era of spacecraft maintenance, enabling autonomous repair systems to address critical issues in space. These systems can identify and diagnose problems with spacecraft components, such as solar panels or thrusters, and carry out repairs without human intervention. This capability is crucial for extending the operational lifespan of satellites and other space-based assets, reducing costs associated with frequent astronaut missions and minimizing the risks involved in space travel.
Addressing the Challenges of Drug Development and Clinical Trials
Improving Efficiency in Preclinical Testing
A critical aspect of drug development is optimizing preclinical testing procedures. This involves streamlining the processes used to evaluate drug candidates' safety and efficacy in animal models. Faster, more efficient methods, such as advanced in vitro models and high-throughput screening technologies, are crucial for reducing timelines and costs while maintaining rigorous scientific standards. Implementing these advancements allows researchers to identify promising candidates earlier, accelerating the entire drug development pipeline.
Robust data generated during preclinical testing is fundamental to informed decisions regarding human clinical trials. The insights gained from these animal studies provide valuable information about potential side effects and optimal dosages, minimizing risks and maximizing the likelihood of success in subsequent human trials. By improving the efficiency of preclinical testing, we can significantly reduce the overall time and resources required for the development of new drugs.
Addressing Ethical Considerations in Clinical Trials
Ethical considerations are paramount in clinical trials. Maintaining the safety and well-being of human participants is paramount, and robust ethical review boards are essential to ensure trials adhere to the highest ethical standards. Transparency in study protocols, informed consent procedures, and data handling practices is critical to fostering trust and accountability. Clear communication with participants throughout the trial is crucial for their comfort and understanding.
Protecting vulnerable populations within clinical trials requires extra attention. Researchers must meticulously consider the potential risks and benefits for individuals who may be more susceptible to harm or less able to provide informed consent. Implementing stringent protocols and safeguards is essential to protecting the rights and well-being of these populations, ensuring that clinical trials serve the wider community while minimizing harm.
Optimizing Trial Design for Robust Data Collection
Well-designed clinical trials are essential for generating reliable data. Careful consideration of the study population, outcome measures, and statistical analysis methods is crucial. Researchers must employ appropriate randomization techniques and blinding strategies to minimize bias and ensure the integrity of the results. The use of sophisticated statistical methods is vital to interpreting complex datasets and drawing meaningful conclusions.
Overcoming Challenges in Patient Recruitment and Retention
Attracting and retaining participants in clinical trials can be a significant hurdle. Effective recruitment strategies, often utilizing a variety of channels, are necessary to reach the target population. Clear communication about the trial's purpose, procedures, and potential benefits is crucial in attracting eligible participants. Creating a positive and supportive trial environment can enhance participant retention and contribute to the successful completion of the study.
Managing the Financial Burden of Drug Development
Drug development is an expensive process. Securing adequate funding and managing costs effectively is critical to the success of the endeavor. Collaboration between pharmaceutical companies, research institutions, and government agencies can help mitigate financial challenges. Exploring innovative funding models, such as public-private partnerships, can help to alleviate the financial burden and accelerate the pace of drug discovery.
Utilizing Technology to Enhance Efficiency and Accuracy
Technology plays a transformative role in drug discovery and clinical trials. Digital tools can streamline data management, improve communication among stakeholders, and enhance the overall efficiency of the process. Electronic data capture systems can reduce errors and improve data quality, leading to more accurate and reliable results. Artificial intelligence and machine learning algorithms can be used to analyze large datasets and identify potential drug candidates and predict patient responses more effectively.
Enhancing Collaboration and Knowledge Sharing
Collaboration among researchers, clinicians, and other stakeholders is essential for advancing drug development and clinical trials. Open communication channels and knowledge-sharing platforms can foster innovation and accelerate progress. Facilitating the exchange of research findings, best practices, and emerging technologies can lead to a more coordinated and efficient drug development process. International collaborations can broaden the scope of research and address global health challenges more effectively.