Integrating Big Data and Computational Modeling
Big Data Applications
Big data, encompassing vast datasets from diverse sources like electronic health records, social media, and genomic sequencing, offers tremendous opportunities for mental health research. Analyzing these comprehensive datasets reveals patterns and potential biomarkers that traditional methods might overlook. This deeper analysis enables better understanding of how genetic, environmental, and lifestyle factors interact in mental illness, potentially speeding identification of new treatment targets.
The massive scale and variety of this information demands advanced computational techniques. Machine learning algorithms can process enormous datasets, detect subtle trends, and forecast drug performance characteristics. This analytical capacity at unprecedented scales helps identify promising compounds faster, potentially yielding more effective, personalized mental health treatments.
Biological Simulation Techniques
Computational models provide powerful tools for simulating biological processes and predicting how potential drugs might interact with the brain's complex systems. By incorporating detailed cellular interactions, molecular mechanisms, and neural pathways, these simulations offer insights into compound effects on specific targets, helping optimize drug design and anticipate side effects.
For mental health applications, these models can simulate drug impacts on neurotransmitter systems, synaptic connections, and brain circuits. This virtual testing capability before expensive clinical trials reduces risks and accelerates development. Ultimately, these modeling advances contribute to discovering more precise mental health treatments.
Connecting Data to Discovery
Combining big data with computational modeling bridges the gap between information and treatment development. This innovative strategy leverages data analysis to uncover critical insights and translate them into practical drug development approaches. Integrating these methods provides deeper understanding of mental illness biology and accelerates progress toward new therapies.
Successful implementation requires collaboration among data scientists, modelers, and clinical researchers. This teamwork ensures comprehensive approaches that transform data insights into practical applications improving patient outcomes.
Clinical Trials and Translational Research

Study Design Principles
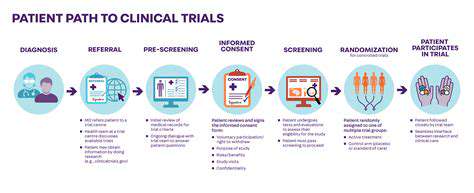
Careful participant selection is fundamental in clinical trials. Enrollment should accurately represent the intended patient population to ensure result applicability. This requires precise definition of eligibility criteria, balancing homogeneity for meaningful comparisons with sufficient diversity to reflect real-world conditions. Methodological rigor is essential to reduce bias and ensure findings validity. Study designs must account for potential confounding variables and employ appropriate statistical analysis methods.
Treatment protocols including dosages, administration schedules, and duration must be clearly defined and consistently implemented. Detailed procedures ensure standardized care and minimize variability. Comprehensive method documentation is crucial for reproducibility and data integrity.
Information Management Approaches
Reliable data collection methods are vital for producing accurate results. Standardized assessment tools like validated questionnaires and clinical scales maintain consistency across participants, enabling objective outcome comparisons. Proper data handling procedures, including management of missing information and entry validation, are essential throughout the study.
Correct statistical analysis is critical for interpreting collected data. Appropriate methods must match the study design and data type to draw accurate conclusions. Analysis should address potential biases and confounding factors to prevent misinterpretation. Understanding analysis limitations is essential for proper interpretation and avoiding overgeneralization. Proper power calculations ensure adequate participant numbers to detect meaningful effects when present.
Ethical Research Practices
Protecting participant welfare is paramount in clinical research. Institutional review boards evaluate study risks and benefits while ensuring protocol compliance with ethical standards. The informed consent process must be thorough and transparent, providing complete information about study procedures, potential risks, and anticipated benefits.
Maintaining participant confidentiality requires strict data security measures. Respecting participants' rights and autonomy throughout the study is fundamental. Researchers must also consider the study's broader societal impacts, conducting work responsibly and ethically.
Implementing Research Findings
Translating clinical trial results into practice is a critical research phase. Ensuring findings are relevant to diverse clinical settings and patient groups is essential. Practical implications must be carefully considered to determine how results can inform clinical decision-making. Effective dissemination educates healthcare professionals and policymakers about study implications.
Developing implementation strategies such as provider education materials or new clinical protocols is crucial. Effective implementation ensures research benefits reach patients and improve outcomes. Ongoing evaluation of implemented strategies identifies areas needing adjustment and measures their real-world impact.