The Crucial Role of Post-Market Surveillance

Post-Market Surveillance and Safety
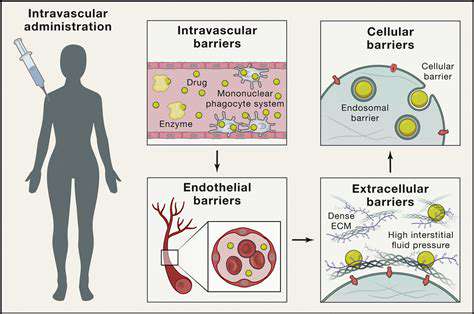
Post-market surveillance plays an indispensable role in guaranteeing the ongoing safety and efficacy of medical devices and similar products after their market release. Through continuous real-world monitoring, manufacturers can uncover and address risks that may have gone unnoticed during initial testing phases. This vigilant process often leads to critical updates or recalls, directly protecting patient health and public safety. Without such oversight, potentially dangerous flaws could remain undetected until they cause harm.
Data Collection and Analysis
The backbone of effective surveillance lies in comprehensive data gathering from multiple sources including patient feedback, healthcare professional reports, and market performance metrics. Pattern recognition through detailed analysis helps pinpoint emerging safety issues or functionality problems that require attention. This ongoing evaluation provides manufacturers with actionable insights to enhance their products' real-world performance and reliability.
Regulatory Oversight and Compliance
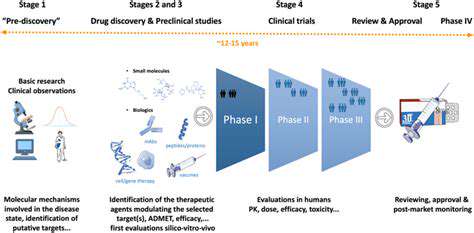
Government agencies establish and enforce stringent guidelines for post-market monitoring procedures. Strict adherence to these regulations maintains public confidence while ensuring products meet established safety benchmarks. Regulatory frameworks create standardized approaches to data collection and reporting, forming a critical safety net for consumer protection. The collaboration between manufacturers and regulators through these processes helps sustain product quality over time.
Product Improvement and Modification
Real-world usage data frequently reveals opportunities for product enhancement that laboratory testing couldn't anticipate. This feedback loop enables manufacturers to make targeted improvements that elevate both safety and performance standards. By continuously refining products based on actual user experiences, companies can stay ahead of potential issues while better serving evolving market needs. Such proactive refinement represents industry best practice for maintaining excellence.
Economic Impact and Efficiency
Well-designed surveillance systems provide substantial financial benefits by identifying problems early. Advanced reporting tools and analytical methods allow for more efficient safety monitoring, reducing the likelihood of expensive recalls. This optimized approach to quality control helps companies allocate resources more effectively while minimizing legal and reputational risks. The economic argument for robust post-market monitoring grows stronger as technologies advance.
Patient and Public Engagement
Direct input from end-users offers invaluable perspectives that traditional data collection might miss. Creating accessible channels for consumer feedback ensures products meet real-world needs while quickly surfacing potential safety concerns. This collaborative approach fosters trust between manufacturers and the public while continuously improving healthcare outcomes. When patients become active participants in safety monitoring, the entire system becomes more responsive and effective.
The Future of Post-Market Surveillance

Post-Market Surveillance and Regulatory Oversight
The evolving landscape of product monitoring demands increasingly sophisticated coordination between regulators and manufacturers. Modern surveillance systems must balance rigorous safety protocols with the flexibility to accommodate rapid technological progress. Emerging tools like AI-driven analytics enable faster detection of potential hazards, while maintaining transparent communication channels ensures timely response to identified risks. Regulatory frameworks must evolve in parallel with product innovation to remain effective.
Forward-thinking agencies are incorporating machine learning to analyze complex data patterns that might indicate safety concerns. Voluntary reporting initiatives encourage manufacturers to share potential issues before they escalate, creating a more proactive safety culture. This collaborative approach helps prevent serious incidents while maintaining public trust in regulated products.
Technological Advancements and Data Analysis
Cutting-edge analytics tools are revolutionizing how we interpret product performance data. The integration of IoT devices provides unprecedented monitoring capabilities, though it requires careful attention to data security and user privacy. Advanced algorithms can now identify subtle usage patterns that may signal emerging problems, enabling preventive action before issues become widespread. These technological solutions complement traditional surveillance methods for more comprehensive protection.
Connected health technologies generate vast datasets that, when properly analyzed, offer deep insights into real-world product performance. Artificial intelligence applications can process these complex datasets to flag potential safety concerns faster than manual review processes. However, the ethical implications of extensive data collection necessitate robust governance frameworks to protect consumer rights while enhancing safety.
Industry Collaboration and Transparency
Modern safety monitoring thrives on open collaboration between all stakeholders in the product lifecycle. Manufacturers who prioritize transparent communication about product performance and potential risks build stronger trust with both regulators and consumers. Detailed documentation of product specifications, proper usage guidelines, and known limitations forms the foundation of responsible post-market practices.
Accountability measures must keep pace with technological complexity. Clear protocols for addressing consumer concerns and disseminating safety information ensure all parties can make informed decisions. This transparent approach not only improves product safety but also strengthens the relationship between companies and the communities they serve.
Consumer Engagement and Feedback Mechanisms
End-users provide some of the most valuable safety data through their firsthand experiences. Digital platforms now enable consumers to easily report concerns through mobile apps, social media channels, and dedicated reporting portals. This direct feedback helps identify real-world issues that laboratory testing cannot predict, creating a more responsive safety ecosystem.
User-friendly reporting systems empower consumers to become active participants in product safety. Simplified reporting processes combined with clear communication about subsequent actions demonstrate a manufacturer's commitment to consumer well-being. By valuing and acting upon user feedback, companies can develop safer products that better meet actual usage needs and expectations.