The Revolutionary Impact of Gene Editing on Tissue Regeneration
Unlocking Tissue Regeneration Through Genetic Precision
Modern medical science stands at the brink of a transformative era with CRISPR-Cas9 technology leading the charge in tissue regeneration. Unlike traditional treatments that merely manage symptoms, this approach targets the root cause by modifying specific genetic sequences. The ability to rebuild damaged cardiac tissue after myocardial infarction or reconstruct neural pathways in spinal injuries could redefine modern medicine's capabilities. Researchers worldwide are currently refining delivery mechanisms to ensure these molecular tools reach their intended targets with unprecedented accuracy.
Cutting-edge studies demonstrate how genetic modifications can stimulate stem cell differentiation and extracellular matrix restoration. What makes this particularly groundbreaking is the potential for creating custom therapeutic regimens based on a patient's unique DNA blueprint. However, successful implementation requires deeper insights into tissue-specific molecular environments and cellular communication networks.
Correcting Genetic Disorders at Their Source
The paradigm shift in treating inherited conditions comes from addressing their genetic origins rather than just alleviating symptoms. Single-gene disorders like cystic fibrosis, where a defective CFTR gene causes systemic complications, may soon be permanently correctable. By precisely editing the mutation, normal protein production can be restored - potentially eliminating the disease's progression.
This technology offers hope for hundreds of monogenic disorders currently lacking definitive treatments. Beyond individual patients, it presents an opportunity to break generational chains of hereditary diseases. Nevertheless, the scientific community emphasizes the need for extensive ethical frameworks, especially regarding modifications affecting reproductive cells.
Navigating the Ethical Landscape of Genetic Interventions
As with any disruptive technology, genetic engineering presents complex moral questions that demand thoughtful examination. A primary concern involves unintended genomic alterations that could create new health issues while attempting to solve existing ones. The scientific response involves developing increasingly precise editing systems and implementing rigorous preclinical testing protocols.
Accessibility represents another critical challenge, as these advanced therapies must not become exclusive to wealthy populations. Establishing transparent public dialogues and inclusive policy development will be crucial for maintaining societal trust in these medical advancements. International collaboration among researchers, ethicists, and policymakers continues to shape responsible innovation in this field.
The Expanding Horizon of Clinical Applications
With numerous trials progressing from laboratory research to human testing, gene editing's therapeutic potential grows increasingly tangible. Current investigations span diverse areas including hematologic disorders, vision restoration, and neurodegenerative conditions. Success hinges on developing reliable delivery mechanisms that can transport genetic tools safely to affected tissues.
Longitudinal studies remain essential to fully understand the durability and safety of genetic modifications over decades. Potential immune reactions to edited cells present another area requiring focused research. Despite these challenges, the technology's capacity to address previously untreatable conditions makes it one of the most promising frontiers in modern medicine.
Advancing Organ Regeneration Through Genetic Engineering
Precision Engineering for Genetic Corrections
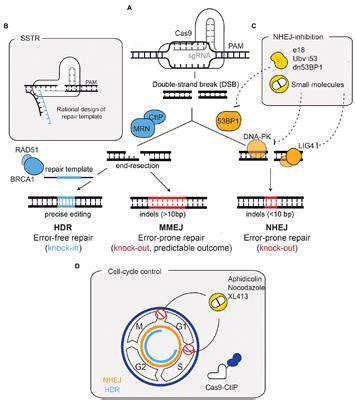
The evolution of CRISPR systems has brought unprecedented accuracy to genetic modifications, enabling corrections at the single-nucleotide level. Such precision is fundamental when editing genes responsible for conditions like sickle cell disease, where a single base mutation causes debilitating symptoms. Current research focuses on enhancing specificity while developing fail-safe mechanisms that prevent unintended DNA alterations. Delivery system innovation remains equally critical, with scientists engineering specialized nanoparticles and viral vectors capable of navigating biological barriers.
This technology's versatility allows for applications across multiple organ systems, from liver regeneration to neurological repair. The personalized medicine aspect becomes particularly valuable when addressing rare genetic variants requiring customized editing approaches.
Balancing Innovation With Ethical Responsibility
As capabilities expand, the scientific community faces increasing responsibility to establish clear boundaries for acceptable applications. Germline editing presents particularly complex questions about humanity's role in directing evolutionary processes. Public understanding and acceptance will largely depend on transparent communication about both potential benefits and limitations.
Equity in access remains another pressing issue, as advanced therapies often carry substantial development costs. Implementing fair distribution systems will be as crucial as the scientific breakthroughs themselves. Regulatory bodies worldwide are working to create frameworks that encourage innovation while protecting against misuse.
Overcoming Technical Hurdles in Implementation
Despite remarkable progress, significant challenges persist in translating laboratory success to clinical practice. Delivery system efficiency varies dramatically across tissue types, with some organs presenting nearly impenetrable biological barriers. Researchers are developing tissue-specific targeting strategies using molecular recognition systems to improve localization.
Minimizing off-target effects requires continuous refinement of guide RNA designs and editor proteins. Advanced computational models now help predict potential unintended edits, allowing for preemptive design adjustments. These improvements are gradually increasing the safety profile of gene editing interventions.
Innovative Delivery Systems for Genetic Therapies
Engineering Targeted Delivery Vehicles
Cutting-edge delivery platforms represent the bridge between genetic tools and therapeutic outcomes. Viral vectors, particularly AAVs, have shown promise in clinical settings but face limitations in cargo capacity. Non-viral alternatives like lipid nanoparticles offer advantages in manufacturing scalability and reduced immune reactivity. The optimal delivery method depends on multiple factors including target organ accessibility, required editing efficiency, and duration of effect.
Emerging technologies include bioengineered exosomes and synthetic polymers designed for organ-specific homing. These platforms incorporate targeting ligands that recognize unique cellular markers, improving localization precision.
Enhancing Precision and Efficiency
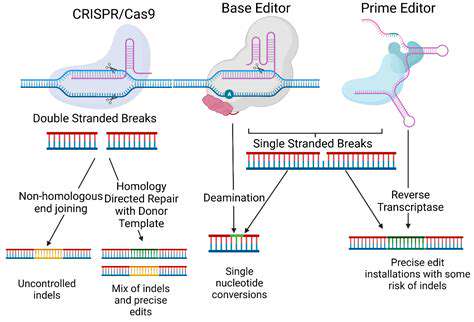
Current research focuses on developing high-fidelity editing systems with reduced off-target activity. Novel CRISPR variants like base editors and prime editors offer more controlled modification approaches. Combining these with advanced delivery mechanisms creates synergistic improvements in therapeutic precision.
Addressing Biological Barriers
Each organ system presents unique challenges for therapeutic delivery. The blood-brain barrier, for instance, requires specialized vehicles capable of neural tissue penetration. Similarly, dense extracellular matrices in certain tissues necessitate delivery systems with enhanced tissue-penetrating capabilities. Researchers are developing solutions ranging from ultrasound-assisted delivery to bioresponsive materials that change properties upon reaching target environments.
Managing Immune Responses
The body's natural defense mechanisms often recognize and attack delivery vectors, limiting therapeutic efficacy. Strategies to overcome this include immune-stealth coatings, temporary immunosuppression protocols, and vector engineering to reduce antigenicity. Understanding individual immune profiles may enable personalized delivery approaches that minimize adverse reactions.
Ensuring Long-Term Safety and Efficacy
Comprehensive monitoring systems are being developed to track edited cells over extended periods. This includes advanced imaging techniques and molecular tracking methods to verify intended edits and detect any aberrant changes. Such monitoring is particularly crucial for therapies intended to provide lifelong benefits.
Scaling Production for Global Access
As therapies advance toward clinical use, manufacturing processes must evolve to meet potential demand. Automation, standardized quality controls, and modular production systems are being implemented to increase output while maintaining consistency. Cost-reduction strategies focus on improving vector yields and developing more efficient purification methods.