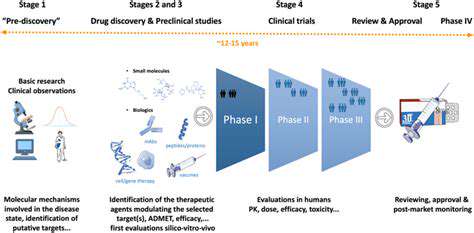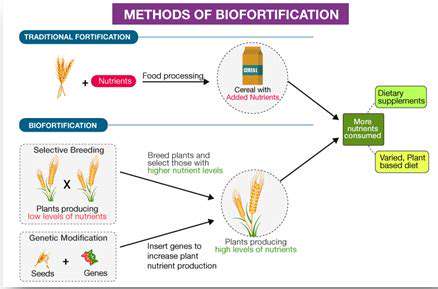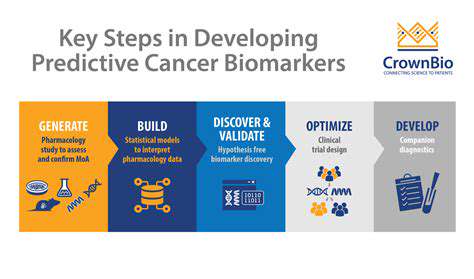
Defining Biomarkers
Biomarkers represent measurable biological indicators that reflect normal processes, pathogenic processes, or pharmacological responses. These indicators span molecular, cellular, genetic, and physiological domains. Accurate biomarker identification forms the foundation for advancing medical knowledge and enhancing clinical practice. They provide critical insights into biological systems, revealing both health status and disease mechanisms.
Effective biomarker identification requires understanding their biological context. For example, inflammatory markers might correlate with specific protein elevations, while genetic variants could indicate disease susceptibility. Reliable biomarker discovery fundamentally supports development of precise diagnostic tools and targeted therapies.
Types of Biomarkers
The biomarker spectrum encompasses diverse forms with unique clinical applications. These range from routine laboratory tests to advanced genomic analyses. Molecular biomarkers, including protein signatures and nucleic acid sequences, have revolutionized disease diagnosis and prognosis assessment. They illuminate disease mechanisms and inform therapeutic decision-making.
Physiological biomarkers, such as cardiovascular parameters, provide valuable health assessments, particularly in preventive medicine and chronic disease management.
Methods for Biomarker Identification
Biomarker discovery employs rigorous scientific methodologies, often involving extensive comparative analyses. Large-scale studies examining samples from diverse populations help establish correlations between biological indicators and disease states. This systematic approach enables identification of meaningful biological patterns with diagnostic or prognostic value.
Validation of Biomarker Candidates
Biomarker validation constitutes a critical phase, confirming consistent association with target conditions. Thorough validation processes ensure biomarker reliability and clinical applicability. This typically requires multiple independent studies to verify initial findings and eliminate false correlations.
Comprehensive validation should include diverse demographic groups to demonstrate broad clinical utility. This establishes the biomarker's relevance across different patient populations and clinical scenarios.
Clinical Applications of Validated Biomarkers
Validated biomarkers enable numerous clinical applications, particularly in early disease detection. Timely diagnosis facilitated by biomarkers can dramatically improve treatment outcomes and survival rates. In oncology, biomarkers assist in tumor classification and staging, directly informing therapeutic strategies.
Additionally, biomarkers monitor therapeutic responses and predict adverse effects, allowing treatment optimization. These capabilities demonstrate biomarkers' transformative potential in contemporary healthcare.
Ethical Considerations in Biomarker Research
Biomarker research necessitates careful ethical deliberation, particularly regarding data privacy and equitable access. Responsible biomarker utilization requires robust ethical frameworks and transparent practices. Researchers must consider potential societal implications while adhering to strict ethical standards throughout all research phases.
Maintaining public confidence demands unwavering commitment to ethical principles in biomarker discovery and application. Transparency and accountability remain essential for sustainable progress in this field.
Applying Biomarkers in Preclinical Studies
Defining Biomarkers in Preclinical Studies
In preclinical research, biomarkers serve as measurable indicators of biological responses to investigational compounds. These indicators prove invaluable for assessing therapeutic potential and safety profiles during early drug development. They span various analytical levels, from basic biochemical assays to sophisticated molecular analyses, providing comprehensive insights into compound effects on biological systems prior to human testing. This critical evaluation phase identifies potential safety issues and informs clinical trial design.
Types of Biomarkers Used
Preclinical studies utilize multiple biomarker categories reflecting different aspects of drug action. These include mechanism-of-action markers, target engagement indicators, and safety parameters. Molecular changes, gene expression patterns, cellular responses, and physiological measurements all contribute valuable data. For instance, quantifying specific protein modifications or transcript levels can elucidate drug-target interactions, while organ function markers help predict potential adverse effects.
Advantages of Using Biomarkers
Biomarker incorporation offers several preclinical research benefits. First, they enable more objective and comprehensive compound evaluation compared to conventional methods. Second, they accelerate development timelines by facilitating early identification of promising candidates and elimination of problematic ones. This optimizes resource allocation throughout the discovery pipeline. Finally, biomarkers provide mechanistic insights that can guide compound optimization and refinement.
Biomarker Selection and Validation
Effective biomarker application requires careful selection and rigorous validation. This process demands thorough understanding of disease pathology and drug mechanisms. Validation establishes the biomarker's relationship to relevant biological outcomes and ensures measurement reproducibility. Such rigorous qualification guarantees data reliability and clinical relevance.
Applications in Different Study Designs
Biomarkers find application across diverse preclinical study formats. In cellular models, they assess compound effects at the molecular level. Animal studies employ biomarkers to evaluate systemic responses and therapeutic efficacy in whole organisms. These applications enable comprehensive compound characterization throughout development, from initial screening to advanced preclinical evaluation.
Challenges and Future Directions
Despite their utility, preclinical biomarker applications face several challenges. These include developing sensitive detection methods, standardizing experimental conditions, and interpreting complex biomarker interactions. Future advancements will likely focus on innovative discovery technologies, integrated data analysis approaches, and predictive modeling to enhance preclinical-to-clinical translation. These developments promise to further improve drug discovery efficiency and success rates.