Beyond the Gene Editing Revolution: Exploring Gene Regulation
While CRISPR dominates discussions about genetic intervention, controlling gene activity levels presents equally promising opportunities. Gene regulation represents biology's sophisticated volume control system, involving intricate interactions between DNA, proteins, and environmental signals. This approach could offer more nuanced therapeutic options by modulating rather than replacing genetic material.
Precision regulation techniques might enable doctors to fine-tune CFTR protein production without permanent DNA changes. Such temporary adjustments could minimize risks while still providing substantial clinical benefits, representing an attractive middle ground between conventional and gene therapies.
Exploring Synthetic Biology: Engineering Biological Systems
The emerging field of synthetic biology applies engineering principles to biological systems. Researchers are designing novel biological components that could potentially bypass defective CFTR channels entirely. These bioengineered solutions might complement or even replace traditional gene editing approaches.
The Role of Epigenetics in Disease and Therapeutics
Epigenetic modifications - chemical tags influencing gene activity without altering DNA sequences - offer another therapeutic avenue. Environmental factors like diet and pollution can modify these epigenetic markers, potentially explaining some CF symptom variability. Targeted epigenetic therapies might reactivate silenced CFTR genes or mitigate disease severity.
Recent studies suggest certain medications can alter epigenetic patterns. This discovery opens possibilities for drug-based epigenetic interventions that could supplement existing CF treatments with fewer side effects than genetic modifications.
Harnessing the Power of Non-Coding RNA
The human genome contains vast regions producing regulatory RNAs that don't code for proteins. These molecules orchestrate complex genetic networks, including those involved in CF pathology. Understanding these RNA controllers could reveal new therapeutic targets for modulating CFTR expression and function.
Some experimental therapies already target microRNAs involved in inflammation, a major CF complication. This approach might complement CFTR-directed treatments by addressing secondary disease aspects.
Targeting the Microbiome for Health and Disease
CF profoundly disrupts respiratory and gut microbiomes, exacerbating symptoms. Novel therapies aim to restore healthy microbial communities through probiotics, phage therapy, or fecal transplants. Microbiome modulation represents a non-genetic approach that could significantly improve patients' quality of life while researchers pursue more permanent genetic solutions.
Challenges and Future Directions in Gene Editing for CF
Off-Target Effects and Safety Concerns
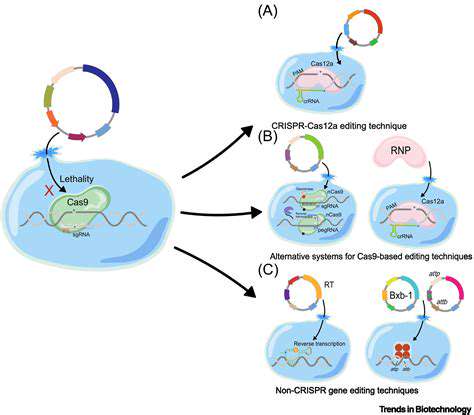
The precision of current gene editing tools remains imperfect, with potential for unintended genetic alterations. These accidental changes could disrupt vital genes or trigger cancerous mutations. Advanced computational modeling and improved editing enzymes are being developed to minimize such risks before human trials can proceed.
Long-term safety monitoring presents another challenge. Unlike conventional drugs that clear from the body, genetic changes are permanent. Decades-long patient follow-up will be essential to fully understand treatment consequences.
Delivery Mechanisms and Cellular Targeting
Effective CF treatment requires delivering editing tools specifically to lung epithelial cells. Current vector systems struggle with inefficient targeting and immune system detection. Next-generation delivery methods including engineered viruses and lipid nanoparticles show promise for overcoming these barriers.
The respiratory tract's complex anatomy complicates uniform treatment distribution. Innovative inhalation technologies combining gene editors with advanced nebulizers could improve delivery efficiency to affected areas.
Ethical Considerations and Public Perception
Germline editing raises profound ethical questions about permanently altering human heredity. While somatic cell editing for CF patients is less controversial, society must establish clear boundaries for genetic interventions. Inclusive public dialogue is crucial to develop consensus on acceptable applications.
Misinformation about genetic technologies remains widespread. Transparent educational initiatives can help patients and families make informed decisions about participating in experimental therapies.
Cost and Accessibility
Current gene therapies carry exorbitant price tags, potentially limiting access. Streamlining production methods and implementing innovative payment models could help democratize these treatments. Global health equity considerations must guide pricing and distribution strategies.
Long-Term Durability and Maintenance of Gene Editing
The durability of genetic corrections remains uncertain. Some edited cells might lose therapeutic effects over time through natural turnover or epigenetic silencing. Ongoing research explores strategies to ensure persistent CFTR expression, including targeting stem cell populations for more permanent solutions.
Clinical Trials and the Road Ahead for CF Patients
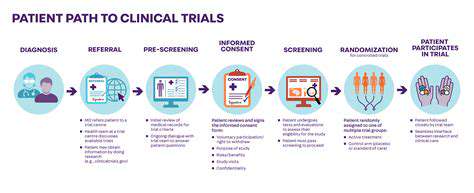
Understanding the Clinical Trial Process
Clinical research follows rigorous protocols to evaluate new treatments objectively. CF trials typically progress through phased studies assessing safety, dosage, and efficacy. Patient participation remains the cornerstone of therapeutic advancement, though enrollment criteria are necessarily strict to ensure data validity.
Ethical Considerations in Clinical Trials
Institutional review boards meticulously evaluate trial designs to protect participants. Informed consent processes have become increasingly comprehensive, ensuring volunteers understand potential risks and benefits. Continuous monitoring safeguards participant welfare throughout studies.
The Role of Data Analysis in Clinical Trials
Advanced analytics transform raw clinical data into meaningful insights. Modern trials incorporate artificial intelligence to detect subtle treatment effects and identify optimal patient subgroups. These analytical innovations accelerate therapeutic development while maintaining scientific rigor.
Challenges and Limitations of Clinical Trials
Rare diseases like CF face particular recruitment challenges due to small patient populations. Decentralized trial models using telemedicine and home monitoring help overcome geographic barriers. Patient advocacy groups play vital roles in connecting researchers with potential participants.
Emerging Trends in Clinical Trial Design
Adaptive trial designs that modify protocols based on interim results are gaining traction. These flexible approaches accelerate evaluation of promising treatments while maintaining safety standards. Digital biomarkers and remote monitoring technologies are revolutionizing data collection.
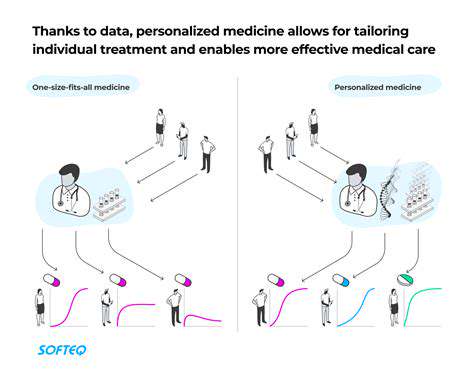
The Future of Clinical Trials and Their Impact
Personalized medicine approaches will increasingly tailor trials to individual genetic profiles. International collaboration expands access to experimental therapies while generating more diverse datasets. These advances promise to shorten the path from laboratory discovery to patient benefit.