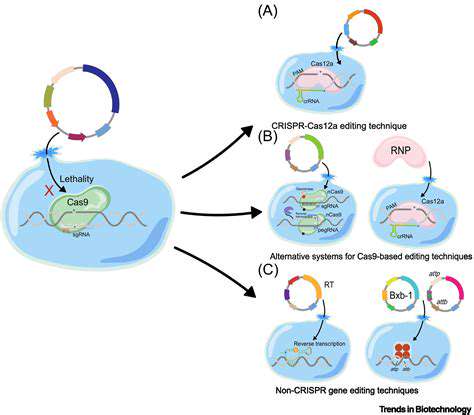
In recent years, the scientific community has witnessed a paradigm shift in genetic engineering with the advent of CRISPR-Cas9 technology. This innovative approach, inspired by bacterial immune defenses, has transformed our capacity to manipulate DNA with surgical precision. What sets CRISPR-Cas9 apart is its remarkable ability to pinpoint and modify specific genetic sequences, offering new hope for treating previously incurable genetic disorders.
Unlike earlier genetic modification techniques, CRISPR-Cas9 combines simplicity with extraordinary versatility. Researchers across the globe are leveraging this tool to accelerate discoveries in diverse fields, from fundamental biological research to pharmaceutical development. The technology's capacity to address complex diseases like cystic fibrosis and various cancers represents one of the most significant medical breakthroughs of our time.
Targeting Specific Genes with CRISPR-Cas9
At the heart of CRISPR-Cas9's effectiveness lies its unique targeting mechanism. The system employs a guide RNA molecule that acts like a molecular GPS, directing the Cas9 enzyme to exact locations within the genome. This precision targeting significantly reduces unintended genetic alterations while maximizing the accuracy of desired modifications - a critical factor for therapeutic applications.
This molecular precision enables scientists to address genetic mutations at their source. By making targeted corrections to faulty DNA sequences, researchers are developing potential cures for hereditary conditions that have eluded medical science for generations.
The technology's versatility is evidenced by its successful application across a broad spectrum of organisms. From microbial systems to human cell lines, CRISPR-Cas9 has demonstrated consistent effectiveness, highlighting its potential as a universal genetic editing platform.
Applications of CRISPR-Cas9 in Medicine
The medical applications of CRISPR-Cas9 are particularly groundbreaking in the realm of genetic disorders. Scientists are making remarkable progress in developing therapies for conditions like sickle cell anemia, where correcting a single genetic mutation could potentially cure the disease permanently.
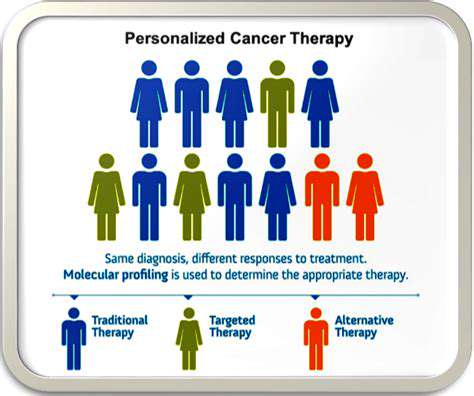
In oncology, researchers are exploring innovative approaches using CRISPR-Cas9 to develop personalized cancer treatments. By precisely targeting cancer-specific genetic markers, this technology could revolutionize how we approach tumor treatment and management.
Beyond direct therapeutic applications, CRISPR-Cas9 is facilitating significant advances in diagnostic development. Researchers are creating novel testing methodologies for infectious diseases and other conditions, potentially enabling earlier and more accurate diagnoses.
Ethical Considerations and Future Directions
The power of CRISPR-Cas9 to modify the human germline presents profound ethical questions that require careful deliberation. As we stand on the threshold of being able to alter inheritable genetic traits, we must establish robust ethical frameworks to guide this technology's responsible application.
Current research efforts are focused on refining CRISPR-Cas9's precision and expanding its therapeutic potential. These advancements must proceed hand-in-hand with ongoing ethical evaluation to ensure the technology benefits humanity while minimizing potential risks.
Clinical Trials and Future Directions
Harnessing CRISPR-Cas9 for Allergy Prevention
CRISPR-Cas9 gene editing technology is opening new frontiers in allergy prevention. Scientists are developing methods to precisely modify genes associated with hypersensitivity reactions, potentially offering permanent solutions for allergy sufferers. Recent studies indicate the technology's effectiveness in disrupting the inflammatory pathways central to allergic responses, enabling customized treatment approaches.
The preventive potential of CRISPR-Cas9 extends beyond symptom management. By targeting immune cell precursors, researchers envision the possibility of stopping allergies before they develop - a groundbreaking shift from treatment to true prevention.
Targeting Specific Allergens and Pathways
Effective allergy prevention requires meticulous identification of genetic targets. Scientists are mapping the complex interplay between genetic factors and immune responses to pinpoint the most promising intervention points.
A primary focus involves genes responsible for IgE antibody production. Modifying these genetic components could potentially prevent the cascade of immunological events that culminate in allergic symptoms.
Ex Vivo Gene Editing and Immune Cell Modification
Ex vivo approaches, where immune cells are modified outside the body before reintroduction, offer significant safety advantages. This method allows for rigorous quality control and minimizes the risk of unintended genetic alterations in the patient.
Safety and Ethical Considerations
The transition from laboratory research to clinical application requires meticulous safety evaluation. Researchers must thoroughly assess potential off-target effects and establish comprehensive monitoring protocols.
Ethical discussions must address the implications of permanent genetic modifications, particularly regarding germline editing. Transparent public dialogue and clear regulatory guidelines are essential for responsible technology deployment.
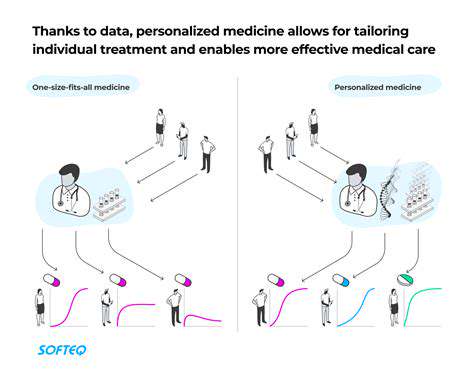
Personalized Medicine and Tailored Approaches
CRISPR-Cas9 enables truly personalized allergy prevention strategies. By analyzing individual genetic profiles, clinicians can develop customized intervention plans that account for each patient's unique genetic predisposition.
This precision approach could dramatically reduce allergy incidence by addressing specific genetic vulnerabilities before symptoms manifest.
Clinical Trial Design and Implementation
Rigorous clinical trial design is crucial for establishing CRISPR-based allergy prevention. Studies must incorporate diverse patient populations and employ standardized assessment protocols to ensure reliable, generalizable results.
Long-term follow-up will be essential to evaluate the durability of genetic modifications and monitor for any delayed effects.