Clinical Trials and the Path Forward
Understanding Clinical Trials in Gene Editing for Sickle Cell Disease
Clinical trials play a pivotal role in assessing the safety and effectiveness of gene editing treatments for sickle cell disease. These studies involve carefully chosen participants and are closely supervised by independent oversight committees. This meticulous process guarantees that any possible hazards are detected and addressed promptly while evaluating the therapy's ability to relieve sickle cell symptoms. The findings from these trials will determine whether gene editing can become a viable treatment option on a larger scale.
Clinical trials progress through distinct stages, each serving a specific purpose. Initial Phase I trials concentrate on establishing the treatment's safety. Phase II trials investigate its potential efficacy, whereas Phase III trials compare the novel gene editing method against current standard treatments. Completing these phases successfully is necessary for obtaining regulatory clearance and making the therapy accessible to patients.
Gene Editing Techniques for Sickle Cell Disease Treatment
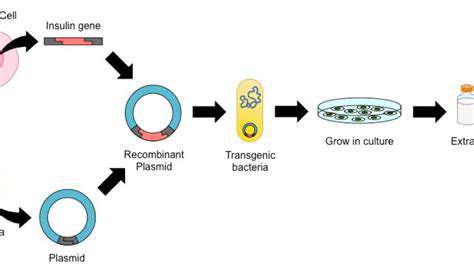
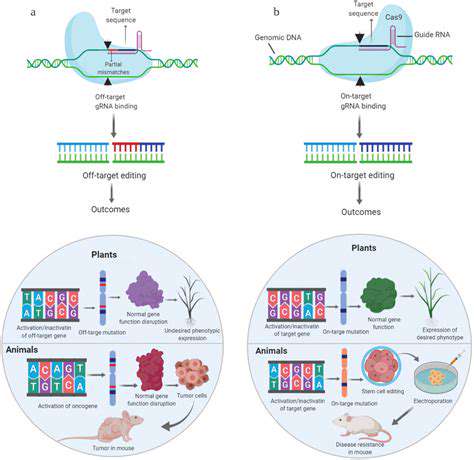
Researchers are examining multiple gene editing approaches for sickle cell disease, each with unique advantages and possible drawbacks. CRISPR-Cas9, a groundbreaking gene editing system, stands out as a leading contender. It enables accurate DNA sequence targeting and alteration, potentially fixing the genetic mutation causing sickle cell disease. Nevertheless, verifying the technique's safety and reliability in clinical applications remains a primary research objective.
Alternative gene editing methods, such as zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs), also show potential. Each technique provides a different way to alter genetic material, and scientists are determining which approach best suits sickle cell disease treatment requirements.
Safety Considerations in Gene Editing Therapies
Ensuring the safety of gene editing treatments is critical. Possible hazards include off-target effects, where unintended genes are modified, and immune system reactions to the altered genetic content. Close observation of trial participants is essential for detecting and addressing these risks promptly. Extensive laboratory testing before human trials helps reduce the chances of such issues arising.
Gathering long-term safety information is equally important. Since gene editing therapies are designed to offer permanent solutions, tracking patients over many years is necessary to fully comprehend the treatment's lasting impact. This information will guide decisions about broader implementation of these therapies.
Ethical Implications of Gene Editing for Sickle Cell Disease
Gene editing presents substantial ethical questions, particularly concerning inheritable genetic changes. Guaranteeing fair distribution of these potentially transformative treatments is imperative. Open public dialogue and transparent policy-making processes are crucial for addressing the ethical challenges surrounding gene editing applications for sickle cell disease.
The Future of Gene Editing in Treating Sickle Cell Disease
While gene editing offers exciting possibilities for sickle cell disease treatment, obstacles persist. Sustained funding for research and clinical trials is vital to enhance the technology, resolve safety issues, and ensure equal access. Cooperation among scientists, medical professionals, and government officials is key to handling ethical dilemmas and enabling broad therapeutic benefits.
Gaining deeper insights into long-term outcomes and optimal delivery methods for gene editing treatments will be essential. Ultimately, progress in this field could transform sickle cell disease management and provide renewed optimism for affected individuals.
Patient Advocacy and Support in Clinical Trials
Patient advocacy organizations significantly contribute to supporting gene editing clinical trials for sickle cell disease. Their participation ensures that patient perspectives inform therapy development and implementation. These groups offer crucial assistance to patients dealing with the complexities of clinical trial participation.
Additionally, advocacy efforts build trust and facilitate communication between researchers, healthcare providers, and communities. Their meaningful involvement helps create a nurturing environment for advancing gene editing therapies and enhancing life quality for sickle cell patients.