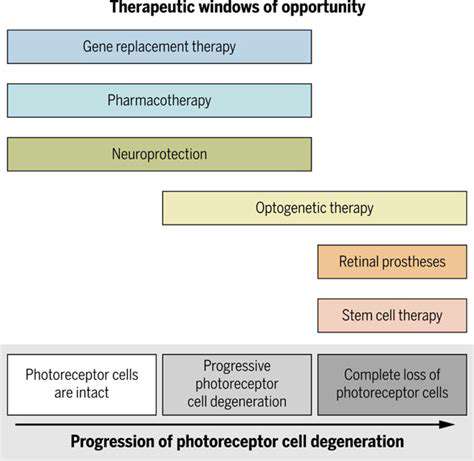
Cutting-Edge Techniques in Retinal Gene Correction
The field of ocular gene correction presents groundbreaking opportunities for addressing genetic vision disorders by targeting their root causes. Unlike traditional treatments that merely manage symptoms, this innovative approach works by supplying healthy genetic material to malfunctioning retinal cells. What makes this technology extraordinary is its potential to not just treat but potentially reverse certain forms of blindness. Scientists are investigating multiple delivery mechanisms, each offering unique benefits for different retinal conditions.
Current methodologies primarily utilize specially engineered viral carriers, particularly adeno-associated viruses (AAVs), known for their safety profile in human applications. The precision of vector selection directly influences treatment outcomes and patient safety. Emerging non-viral alternatives, including nanoparticle systems, show promise for scenarios where viral vectors might not be ideal, potentially reducing immune reactions while maintaining therapeutic effectiveness.
Precision Targeting of Retinal Genetic Defects
Genetic vision disorders stem from mutations affecting various components of retinal function. Successful treatment hinges on identifying the exact genetic culprit in each case. For example, defects in the RPE65 gene disrupt critical cellular processes in the retinal pigment epithelium, leading to progressive vision loss. Corrective genetic interventions targeting this specific mutation have shown remarkable results in clinical settings.
Other therapeutic targets include genes responsible for photoreceptor function (like rhodopsin) and retinal metabolic processes. The complexity of retinal biology demands customized approaches for each genetic variation. This personalized strategy represents the future of ophthalmic medicine, moving beyond one-size-fits-all solutions.
Progress in Human Trials and Emerging Possibilities
Ongoing clinical studies continue to demonstrate the real-world potential of genetic vision correction. These carefully monitored trials provide crucial data on both effectiveness and safety profiles. Initial outcomes suggest significant improvements in visual function for some participants, offering hope for conditions previously considered untreatable.
The next frontier involves refining delivery methods for greater precision and durability of effect. Researchers are particularly focused on developing safeguards against potential immune reactions, a critical consideration for long-term success. The advent of patient-specific genetic customization promises to further enhance treatment accuracy and outcomes in coming years.
Balancing Innovation with Ethical Responsibility
The emergence of genetic vision correction raises important questions about equitable access and responsible implementation. As with any transformative medical technology, thoughtful ethical frameworks must guide its application to ensure broad societal benefit.
From a public health perspective, successful genetic interventions could significantly reduce the personal and economic burden of retinal disorders. This technology may decrease reliance on expensive supportive care while dramatically improving patients' quality of life. Looking ahead, the potential to prevent these conditions in future generations could fundamentally change our approach to inherited blindness.
Modern supply chains are undergoing a radical transformation thanks to blockchain technology. This innovative approach provides an immutable record of product journeys, from raw materials to end consumers. The technology's ability to create transparent, tamper-proof records significantly reduces opportunities for fraudulent activities. By establishing complete product histories, businesses can guarantee authenticity while streamlining operations - creating supply networks that are simultaneously more reliable and efficient.
Overcoming Obstacles in Ocular Gene Therapy Implementation
Navigating Delivery Complexities
The eye's unique anatomical features present distinct challenges for therapeutic delivery. Specialized barriers like the blood-retinal interface require innovative solutions to ensure genetic material reaches its intended destination. Current efforts focus on engineering advanced carriers capable of precise targeting while minimizing unintended effects. This research involves detailed study of cellular uptake mechanisms and optimization of delivery conditions.
While viral vectors show effectiveness, their potential to trigger immune responses remains a concern. Alternative non-viral systems are being developed to provide safer options with comparable delivery efficiency.
Ensuring Treatment Safety
Patient safety remains the foremost consideration in developing genetic vision treatments. Potential immune reactions to either the therapeutic genes or their carriers require careful monitoring. Comprehensive testing protocols help identify and mitigate possible adverse effects before human application.
Current research explores methods to reduce immune responses, including vector modifications and complementary immune modulation. Addressing concerns about unintended genetic alterations remains a priority as the field advances.
Sustaining Long-Term Benefits
The durability of genetic corrections represents a critical factor in treating chronic retinal conditions. Researchers are investigating various approaches to maintain therapeutic effects, including optimized genetic promoters and advanced editing techniques.
Extended patient monitoring provides valuable insights into the longevity of treatment effects and their impact on retinal health over time.
Improving Accessibility
The significant costs associated with developing personalized genetic treatments pose challenges for widespread availability. Innovations in production techniques aim to reduce expenses while maintaining quality standards.
Comprehensive economic assessments must balance upfront costs against potential long-term savings from reduced disease burden and improved patient outcomes.
Navigating Ethical Landscapes
Genetic vision correction raises important questions about the boundaries of medical intervention. Establishing clear ethical guidelines ensures responsible development and application of this powerful technology.
Open dialogue with all stakeholders helps address concerns while maintaining public trust in these groundbreaking treatments.
Advancing Through Rigorous Clinical Evaluation

Designing Effective Clinical Studies
Well-structured clinical research forms the foundation for evaluating new treatments. Thoughtful participant selection and rigorous methodology are essential for generating reliable data. Standardized protocols ensure consistency across study sites while allowing for meaningful analysis of results.
Clear definition of study objectives and endpoints provides focus throughout the research process, from design through implementation.
Regulatory Evaluation Pathways
Medicines regulators employ a phased approach to assess new treatments, progressing from initial safety tests to large-scale efficacy studies. This systematic evaluation protects patients while facilitating medical innovation.
Transparent documentation and thorough analysis support informed regulatory decisions about treatment approvals.
Maintaining Ethical Standards
Protecting research participants requires strict adherence to ethical principles. Comprehensive informed consent processes and data protection measures are non-negotiable requirements.
Independent oversight committees provide essential review and monitoring throughout the research process.
Ensuring Data Integrity
Robust data management systems support accurate collection and analysis of research findings. Predefined statistical plans help avoid bias while ensuring appropriate interpretation of results.
Quality control measures maintain confidence in study outcomes and their implications for patient care.
Navigating Approval Processes
Successful regulatory submissions require complete documentation of research methods and outcomes. Clear communication with regulatory agencies facilitates efficient review of new treatment applications.
Timely responses to regulatory queries help maintain momentum in the approval timeline.
Post-Approval Monitoring
Ongoing surveillance after treatment approval provides critical safety information. This long-term monitoring helps identify any rare or delayed effects not seen in clinical trials.
Continuous safety reporting ensures healthcare providers have current information to guide treatment decisions.
Driving Medical Innovation
Clinical research serves as the bridge between laboratory discoveries and patient treatments. The insights gained from well-designed studies directly shape the future of medical care.
As research methodologies evolve, they enable development of increasingly effective and safer therapies for patients worldwide.