Harnessing the Power of CRISPR-Cas
Originally discovered as part of bacteria's immune defense mechanism, the CRISPR-Cas system has completely transformed modern genetic engineering. Researchers can now make precise modifications to DNA sequences with remarkable accuracy, opening doors to groundbreaking discoveries. This level of precision proves indispensable across various fields, from investigating disease mechanisms to creating innovative treatments. The technology provides an unparalleled opportunity to explore gene functions and potentially rectify genetic abnormalities responsible for diseases.
What makes the CRISPR-Cas system particularly valuable is its programmable nature using guide RNA molecules to pinpoint exact DNA locations. This adaptability enables targeted genetic modifications in diverse organisms, ranging from agricultural crops to human cell lines. Such flexibility positions it as an essential instrument for unraveling intricate biological pathways and formulating precise medical interventions.
Applications and Future Directions
The practical applications of CRISPR-Cas technology already demonstrate extraordinary potential across multiple disciplines. In therapeutic contexts, it offers promising solutions for addressing genetic conditions. Consider the possibility of rectifying inherited disorders such as sickle cell anemia or muscular dystrophy at their genetic roots. This represents a paradigm shift that could redefine medical treatment approaches entirely.
Beyond human health applications, CRISPR-Cas technology shows significant promise for agricultural innovation. Researchers are employing it to cultivate crops with improved nutritional profiles and enhanced resistance to drought and pests. These developments could contribute substantially to global food security while promoting sustainable farming practices. Ongoing research and development efforts remain crucial for fully realizing this technology's transformative capabilities.
Current investigations are exploring CRISPR-Cas applications in oncology and diagnostic procedures. Scientists are examining its potential for selectively targeting malignant cells while sparing healthy tissue. Additionally, CRISPR-based diagnostic platforms may enable quicker and more precise disease identification, representing a major advancement in preventive healthcare and clinical management.
While the scientific community celebrates these advancements, it's important to acknowledge the considerable ethical questions surrounding CRISPR-Cas technology. As the field progresses, establishing frameworks for responsible implementation will ensure we maximize benefits while minimizing potential risks. This requires sustained collaboration between researchers, ethicists, and regulatory bodies.
The trajectory of CRISPR-Cas technology appears exceptionally promising, with its influence across multiple sectors becoming increasingly evident. Sustained investment in research promises to yield even more innovative applications and technological breakthroughs in the coming years.
Beyond Traditional Methods: Advantages of CRISPR Diagnostics
Enhanced Sensitivity and Specificity
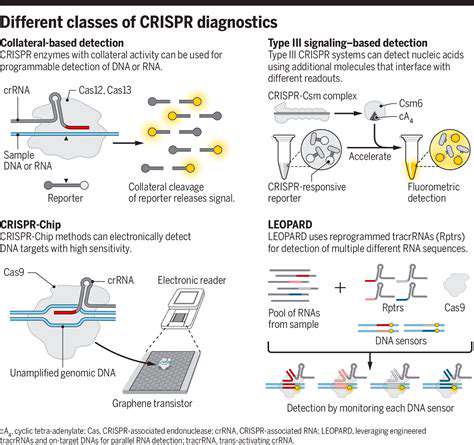
CRISPR-based diagnostic platforms represent a quantum leap in detection capabilities compared to conventional techniques. The system's molecular precision enables identification of minute genetic traces, facilitating earlier and more reliable disease detection—particularly valuable for cancer and infectious disease diagnosis. This enhanced sensitivity dramatically reduces false negative results, providing clinicians with more dependable data for treatment decisions.
Moreover, CRISPR diagnostics can be engineered to recognize specific genetic sequences with extraordinary accuracy, virtually eliminating interference from similar but irrelevant sequences. This specificity proves invaluable when distinguishing between closely related pathogens or genetic variants, resulting in more accurate diagnoses and tailored treatment plans. The technology's capacity to selectively amplify target sequences significantly boosts diagnostic reliability.
Rapid Turnaround Time
Among CRISPR diagnostics' most compelling benefits is their substantially faster processing time relative to traditional methods. Where conventional tests might require days for results, CRISPR-based assays can frequently deliver answers within hours—sometimes even minutes. This speed proves particularly valuable in emergency medical situations and during infectious disease outbreaks where timely diagnosis directly impacts treatment outcomes.
The rapid processing stems from CRISPR's efficient target sequence amplification and detection mechanisms. This streamlined approach enables swift pathogen or genetic mutation identification, facilitating prompt medical interventions and improved patient prognoses. The technology's speed represents a substantial advantage in clinical settings where time is critical.
Cost-Effectiveness
While initial CRISPR technology investments may appear significant, the long-term economic benefits of CRISPR diagnostics are becoming increasingly apparent. Potential for high-volume testing, reduced reagent expenses, and process automation could ultimately lower per-test costs compared to conventional methods—particularly advantageous for large-scale screening initiatives and resource-limited environments.
CRISPR's multiplexing capability—simultaneously detecting multiple targets in a single reaction—further enhances cost efficiency by eliminating the need for separate tests. The technology's scalability and automation potential suggest significant healthcare cost reductions in the long term.
Versatility and Multiplexing
CRISPR technology demonstrates remarkable adaptability, supporting diagnostic assay development for numerous applications. Beyond pathogen detection, it can identify disease-associated genetic mutations spanning oncology, hereditary conditions, and drug metabolism studies. This versatility makes CRISPR an invaluable diagnostic tool with wide-ranging applications.
The technology's multiplex capacity—detecting multiple targets simultaneously in a single sample—greatly enhances testing efficiency. This comprehensive approach provides a more complete health assessment from a single assay, significantly increasing diagnostic value and clinical impact.
Minimally Invasive Sample Collection
Many CRISPR diagnostic platforms utilize non-invasive or minimally invasive sampling methods—a substantial improvement over traditional techniques requiring blood draws or biopsies. Using saliva, nasal swabs, or urine samples improves patient comfort and compliance, especially important for pediatric cases or needle-phobic individuals.
This reduced invasiveness also benefits regions with limited healthcare infrastructure where sophisticated lab equipment may be unavailable. CRISPR diagnostics' compatibility with various sample types greatly enhances its accessibility across diverse healthcare settings, making advanced diagnostics available to broader populations.
Genetic Testing and Disease Screening: Personalized Medicine's New Frontier
Understanding the Role of Genetic Testing in Disease Screening
Genetic analysis is increasingly becoming fundamental to modern disease screening protocols, providing valuable insights into individual disease susceptibilities. By examining a person's unique genetic blueprint, medical professionals can identify potential health risks long before symptoms emerge. This proactive strategy enables earlier medical interventions and potentially more effective treatment approaches. Understanding disease-specific genetic variations allows for customized treatment regimens tailored to each patient's genetic profile, representing a shift toward truly personalized healthcare. This individualized methodology marks a departure from generalized treatment protocols toward more nuanced patient care.
The value of genetic testing extends well beyond simple risk evaluation. It provides crucial information about medication efficacy and potential adverse reactions. Certain genetic variants influence drug metabolism rates, affecting optimal dosing and potential side effect profiles. This knowledge enables physicians to prescribe medications more appropriately, minimizing complications while maximizing therapeutic benefits. This precision medicine approach is transforming treatment paradigms for numerous conditions including malignancies, cardiovascular diseases, and neurological disorders. By elucidating the genetic foundations of these diseases, we can develop more targeted and efficient treatment strategies.
CRISPR Technology's Potential in Genetic Testing and Disease Screening
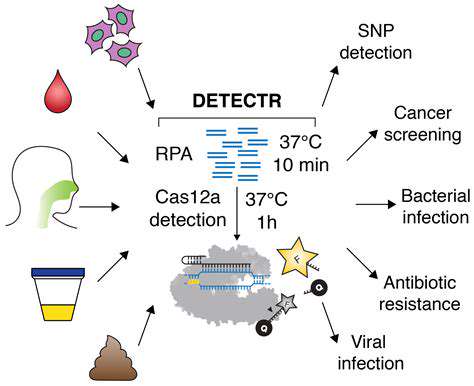
The emergence of CRISPR technology has created exciting opportunities for advancing genetic analysis and disease screening. This revolutionary gene-editing tool enables scientists to precisely locate and modify specific genes, potentially offering new diagnostic and therapeutic avenues for genetic disorders. By facilitating precise identification of disease-associated genetic mutations, CRISPR-based diagnostics promise faster and more accurate results than conventional methods. This innovation could fundamentally transform healthcare by enabling earlier detection of genetic predispositions to various medical conditions.
Ongoing CRISPR research may lead to novel diagnostic tools capable of detecting subtle genetic variations linked to disease risk much earlier in life. Such early detection could facilitate preventive measures that might forestall disease development. Additionally, CRISPR may enable personalized therapies through targeted genetic corrections, potentially offering cures for certain genetic disorders. This revolutionary potential could reshape our approach to human health management.
Integrating CRISPR technology into disease screening protocols promises more rapid, accurate, and affordable genetic testing. This could increase testing accessibility, potentially improving health outcomes at both individual and population levels. Early identification of genetic risks would allow for preventive health measures, shifting healthcare focus toward proactive rather than reactive medicine. This evolving paradigm holds tremendous promise for enhancing global health and wellbeing.
Challenges and Future Directions: Ensuring Accessibility and Scalability
Overcoming Existing Limitations
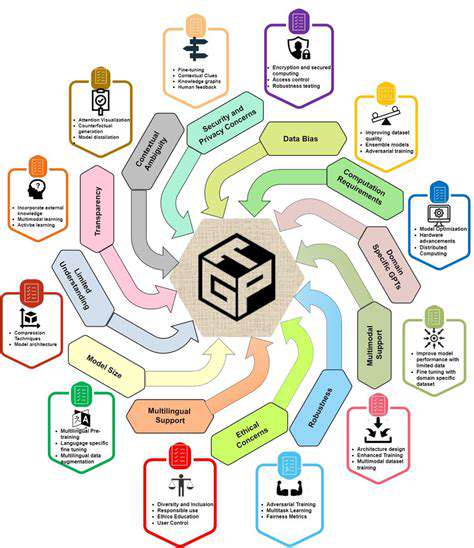
The field currently faces significant challenges regarding standardized datasets for model training and evaluation. This lack of standardization complicates cross-study comparisons and hinders development of robust, widely applicable solutions. Additionally, existing methodologies often falter when confronted with real-world complexities involving incomplete, noisy, or biased data. Addressing these limitations proves essential for broader technology adoption and practical implementation.
Computational resource requirements present another major obstacle. The substantial data volumes and algorithmic complexity frequently demand powerful hardware and significant infrastructure, creating barriers for many research institutions. Developing more efficient algorithms and leveraging cloud computing resources will be crucial for overcoming these challenges.
Data Acquisition and Preprocessing
Model performance depends heavily on data quality and quantity. Obtaining representative, high-quality data that accurately reflects target populations requires meticulous collection strategies and careful consideration of potential biases. This process necessitates thoughtful planning and execution to ensure data integrity.
Effective data preprocessing methods are equally critical for addressing data imperfections. Robust techniques for handling missing values, noise reduction, and data normalization are essential for ensuring model reliability and accuracy. These preprocessing steps form the foundation for successful model development.
Model Selection and Evaluation
Selecting appropriate machine learning models for specific applications significantly impacts performance outcomes. Matching model characteristics to data properties and problem requirements enables more informed selection decisions. Multiple model types exist, each with distinct advantages and limitations, making context-specific choices essential.
Comprehensive evaluation metrics are necessary for accurately assessing model performance. These metrics should meaningfully reflect the model's real-world impact on the addressed problem. Inappropriate metric selection can lead to misleading conclusions and impede technological progress.
Interpretability and Explainability
Many advanced machine learning models—particularly deep learning architectures—operate as black boxes, obscuring their decision-making processes. This lack of transparency can hinder real-world adoption due to trust concerns. Developing models that balance accuracy with interpretability represents a critical research direction.
Efforts to improve model explainability—through visualization techniques and model-agnostic approaches—are essential for understanding model behavior and building user confidence. Transparent models empower users to comprehend and trust predictions, facilitating broader adoption and better implementation outcomes.
Ethical Considerations
Machine learning applications raise important ethical questions regarding fairness, bias mitigation, and data privacy. Models trained on biased datasets risk perpetuating or exacerbating existing societal inequities. Ensuring fairness in both data collection and model development is paramount for ethical technology deployment.
Data privacy concerns also demand serious attention. Protecting sensitive information and maintaining regulatory compliance require robust data anonymization protocols and strict access controls. Implementing comprehensive privacy safeguards is essential for addressing these ethical challenges.
Computational Resources and Scalability
Increasing model complexity creates substantial computational demands for both training and deployment. Developing efficient algorithms and optimized hardware solutions is crucial, especially for large-scale implementations.
Scalability considerations are equally important to accommodate growing datasets and increasing prediction demands. Leveraging cloud platforms and distributed computing frameworks can help address these scaling challenges effectively.