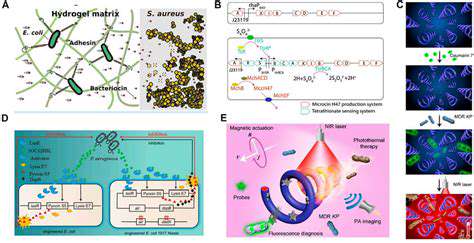
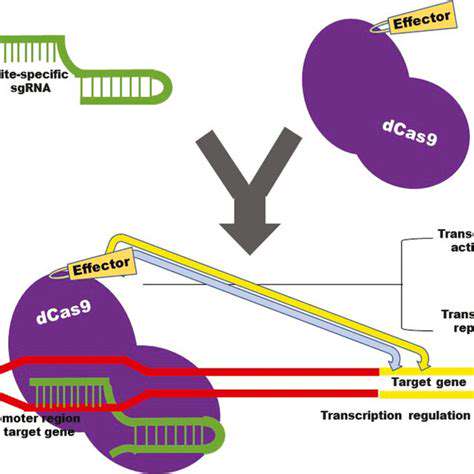
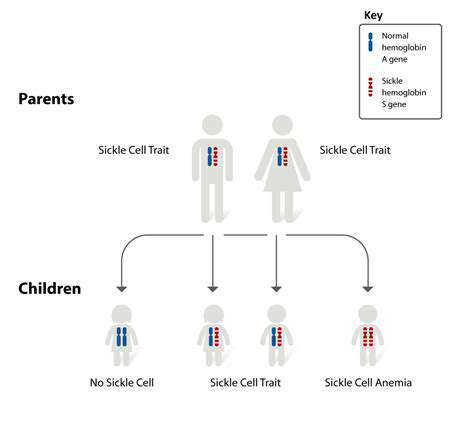
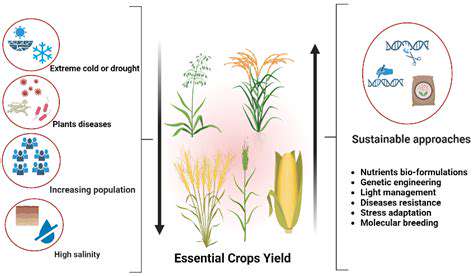
Enzymes, as biological accelerators, play a pivotal role in speeding up biochemical reactions within living systems. Their extraordinary specificity and catalytic efficiency render them perfect instruments for environmentally friendly applications in synthetic biology. Through millions of years of evolution, these protein molecules have been optimized to execute specialized functions with minimal byproduct generation. Capitalizing on these natural capabilities facilitates the creation of more sustainable chemical processes that reduce dependence on aggressive reagents and hazardous waste products.
While industrial enzyme use isn't novel, recent breakthroughs in protein modification and bioreactor technology have dramatically broadened their potential applications. Modern scientists can now adapt existing enzymes or engineer novel variants with customized characteristics, enabling them to facilitate reactions under gentler conditions, utilize renewable resources, and generate valuable chemical compounds with improved efficiency and reduced ecological consequences.
Utilizing Microbial Metabolic Capabilities
Microorganisms harbor extensive metabolic networks that can be leveraged for eco-friendly transformations. Ranging from bacterial species to fungal organisms, these lifeforms demonstrate remarkable proficiency in converting diverse raw materials into useful end products. Through careful study and modification of these metabolic routes, synthetic biologists can develop microbial production platforms capable of manufacturing chemicals, fuels, and materials with significantly reduced environmental consequences.
This strategy offers multiple benefits compared to conventional chemical synthesis methods. Microbial processes typically function under mild environmental conditions, eliminating the necessity for extreme temperatures or pressures. Additionally, incorporating renewable starting materials like agricultural byproducts or atmospheric carbon dioxide can help establish more sustainable manufacturing systems.
Tailoring Biocatalysts for Specific Applications
A crucial component of enzyme utilization for sustainable transformations involves the development of specialized biocatalysts for particular chemical reactions. This process requires meticulous selection or genetic modification of enzymes to enhance their catalytic properties, substrate specificity, and operational stability. Representative examples include engineering enzymes to withstand harsh industrial conditions, expand their reactivity spectrum, or increase their efficiency within specific reaction parameters.
Enhancing Biocatalyst Durability and Recyclability
Enzymes frequently exhibit sensitivity to environmental fluctuations in temperature, acidity, and chemical exposure. Improving their operational stability and reuse potential is essential for industrial implementation. Stabilization approaches include immobilization techniques, where enzymes are affixed to solid matrices, and strategic amino acid modifications that increase structural integrity. These adaptations permit multiple usage cycles, reducing waste generation and improving economic feasibility—critical factors in establishing genuinely sustainable industrial processes.
Incorporating Biocatalysis into Conventional Chemical Manufacturing
The effective adoption of biocatalytic methods in synthetic biology depends on smooth integration with established chemical processes. Researchers are developing innovative strategies to seamlessly combine enzymatic reactions with conventional synthetic steps. This integration can yield more efficient and ecologically sound production pathways for various chemical products and materials. Comprehensive optimization of reaction parameters and process architecture is necessary to maximize biocatalysis benefits while mitigating potential limitations.
Developing intuitive navigation requires anticipating user actions and crafting pathways that align with their natural thought processes. The interface design should clearly convey its organizational structure, ensuring users never experience confusion or disorientation. Platform visitors should intuitively grasp how to navigate between different sections without resorting to trial-and-error exploration.
Optimization and Scale-Up of Bioprocesses
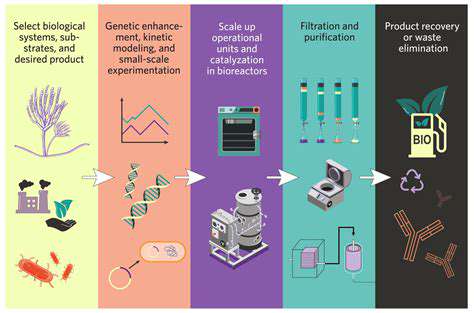
Strategic Approaches to Bioprocess Enhancement
Bioprocess refinement is essential for maximizing output and efficiency in biological production systems. This requires a comprehensive strategy involving precise regulation of multiple variables including environmental temperature, acidity levels, and nutrient availability. Strategic adjustment of these parameters can dramatically improve microbial proliferation and target compound synthesis. Employing sophisticated experimental frameworks, such as factorial designs or response surface analysis, proves invaluable for determining optimal operating conditions and deciphering variable interactions. This methodical process reduces unnecessary experimentation and accelerates the identification of ideal operational parameters.
Scale-Up Challenges in Bioreactor Implementation
Transitioning bioprocesses from laboratory scale to industrial production introduces distinctive obstacles. Consistent performance maintenance and product quality preservation across different operational scales remains a primary concern. Significant attention must be directed toward the engineering and management of larger bioreactor systems, incorporating considerations for mixing efficiency, oxygen delivery mechanisms, and thermal regulation. Various bioreactor configurations—including agitated vessels, airlift designs, and packed-bed systems—each present unique advantages and limitations regarding scalability. Thoughtful selection and optimization of the most suitable reactor type is fundamental for successful process expansion.
Operational Variables Affecting Bioproduct Output
Numerous operational factors exert substantial influence on biological product yields. Environmental conditions including temperature, pH balance, and nutrient concentration can either optimize or disrupt critical metabolic processes in production organisms. Careful optimization of these parameters enables maximization of desired product formation. Additionally, oxygenation levels and agitation intensity significantly impact microbial growth and product synthesis by ensuring adequate oxygen availability. Proper adjustment of these operational variables can produce marked improvements in overall process performance.
Microbial Strain Selection and Genetic Modification
The selection of appropriate microbial strains represents a crucial decision in bioprocess development. Identifying organisms with favorable traits—such as high productivity, environmental robustness, and cultivation simplicity—is essential for successful implementation. Genetic manipulation techniques can further augment the production capabilities of selected strains. Introducing specific genetic elements or modifying existing biochemical pathways can yield substantial increases in product formation and overall process efficiency.
Upstream Processing and Initial Purification
Initial processing stages encompassing cell cultivation and harvest constitute fundamental components of bioprocess optimization. Effective cell culture and harvesting protocols are necessary for cost containment and yield maximization. Efficient techniques for biomass separation and concentration are critical for subsequent purification stages. Furthermore, maintaining strict sterility throughout these operations is vital for preventing product degradation and ensuring final product quality.
Downstream Processing and Product Refinement
Downstream operations focused on purifying target compounds from fermentation broths demand meticulous attention. Advanced separation and purification technologies—including various chromatographic methods and precipitation techniques—are essential for achieving high-purity products. These methodologies require careful optimization to ensure maximum product recovery with minimal losses. Such optimizations are crucial for maintaining economic viability and ensuring product specifications are met.
Economic and Environmental Sustainability Factors
Financial feasibility and ecological sustainability represent critical considerations in bioprocess development and implementation. Comprehensive evaluation of production costs—incorporating raw material expenses, labor requirements, and energy consumption—is necessary for assessing commercial viability. Adoption of sustainable practices, including waste reduction strategies and renewable resource utilization, is essential for long-term success. Environmentally conscious bioprocesses can make significant contributions to the establishment of greener industrial practices.