Understanding the Role of Model Organisms
Model organisms—including fruit flies, zebrafish, and mice—serve as indispensable tools for developmental research. Their simplified genetics, rapid generation times, and manageable lifespans facilitate controlled studies of complex biological phenomena. Such experimental systems have significantly advanced our comprehension of human development and disease mechanisms.
When combined with gene editing, model organisms permit targeted genetic modifications, enabling detailed observation of developmental consequences. This approach yields profound insights into gene functionality and its influence on growth, differentiation, and morphological patterning.
Gene Editing Techniques in Model Organisms
Modern gene editing methodologies like CRISPR-Cas9 have revolutionized developmental biology research. These tools facilitate exact genetic alterations in model organisms, permitting systematic investigation of gene disruption effects. The precision of contemporary editing techniques has dramatically enhanced our ability to probe specific gene functions within developmental cascades.
Applying these methods in model systems creates powerful platforms for examining gene interactions and their developmental impacts. Engineered organisms with specific genetic modifications allow controlled functional studies under defined experimental conditions.
Investigating Developmental Pathways in Zebrafish
Zebrafish embryos offer unique advantages for developmental studies due to their optical clarity, enabling real-time observation of organogenesis. This visual accessibility, combined with genetic manipulation, provides unparalleled insights into developmental regulation mechanisms.
Zebrafish-based research using gene editing has uncovered critical gene functions in organ and tissue development, findings with significant implications for understanding human developmental disorders and potential treatment strategies.
Exploring Developmental Processes in Fruit Flies
Drosophila melanogaster serves as an exemplary model for fundamental developmental studies. Their straightforward genetics and rapid lifecycle make them ideal for investigating basic processes like cell fate determination. Gene editing enables precise manipulation of developmental genes, facilitating functional analysis.
Dissecting Developmental Defects in Mice
Mouse models provide critical insights into complex developmental processes relevant to human biology. Advanced genetic tools combined with editing technologies allow creation of mice with targeted mutations, enabling detailed study of developmental abnormalities and disease phenotypes. The genetic complexity of mice permits investigations closely mirroring human developmental biology.
Ethical Considerations and Future Directions
While gene editing in model organisms offers tremendous scientific potential, it necessitates careful ethical consideration. Research applications must adhere to stringent ethical guidelines, particularly regarding potential human germline applications.
Future progress depends on developing more precise editing tools and analytical methods for studying gene interactions. Interdisciplinary collaboration between biologists, geneticists, and ethicists will be essential for responsible advancement in this rapidly evolving field.
Potential Therapeutic Applications: Repairing Developmental Defects
Gene Therapy for Genetic Disorders
Gene therapy represents a transformative approach for treating genetic conditions by addressing their fundamental genetic causes. This strategy aims for permanent genetic correction in affected cells, potentially providing definitive solutions for previously untreatable disorders. Current research explores various delivery mechanisms, including viral vectors and alternative systems, with early clinical trials showing promising outcomes.
Cancer Immunotherapy
Immunotherapeutic approaches are reshaping cancer treatment paradigms by harnessing the immune system's natural capabilities. These targeted strategies often demonstrate superior efficacy and reduced toxicity compared to conventional therapies. Diverse immunotherapeutic modalities—including checkpoint inhibitors and cellular therapies—offer customized approaches for different cancer types.
The evolution of personalized immunotherapies tailored to individual patients promises to further enhance treatment effectiveness while minimizing adverse effects.
Regenerative Medicine
Regenerative medicine focuses on restoring damaged tissues through innovative biological approaches. Stem cell technologies, tissue engineering, and advanced bioprinting methods show particular promise for conditions like cardiovascular disease and neurological damage. While significant progress has occurred in developing supportive scaffolds and growth factors, achieving consistent regeneration in complex tissues remains challenging.
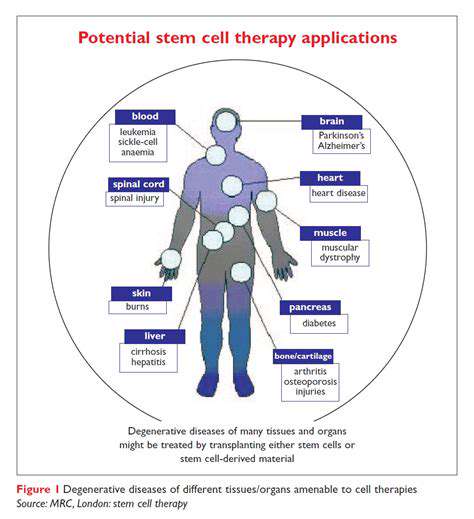
Stem Cell Therapies
Therapeutic applications of stem cells span numerous medical conditions due to their remarkable differentiation potential. These cells offer unprecedented opportunities for tissue repair and regeneration in neurological, cardiovascular, and autoimmune disorders. Ongoing clinical trials continue to evaluate safety and efficacy parameters to optimize therapeutic protocols.
Neurological Disorders
Addressing neurological diseases requires innovative approaches targeting their underlying pathophysiology. Emerging strategies combining genetic and cellular therapies show particular promise for conditions like Parkinson's and Alzheimer's diseases. Effective treatment development necessitates integrated approaches combining pharmacological and non-pharmacological interventions.
Drug Delivery Systems
Advanced drug delivery technologies are revolutionizing therapeutic administration by enhancing targeting precision. Nanotechnology-based systems enable localized drug concentration, improving efficacy while reducing systemic side effects. These platforms also facilitate personalized treatment regimens tailored to individual patient requirements.
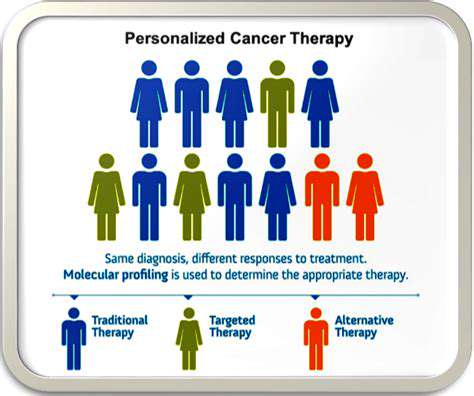
Personalized Medicine
The personalized medicine paradigm represents a fundamental shift in healthcare delivery. By integrating genetic, lifestyle, and environmental data, clinicians can develop customized treatment strategies with optimized effectiveness and tolerability. Genomic and computational advances are accelerating implementation across diverse medical specialties, from oncology to chronic disease management.