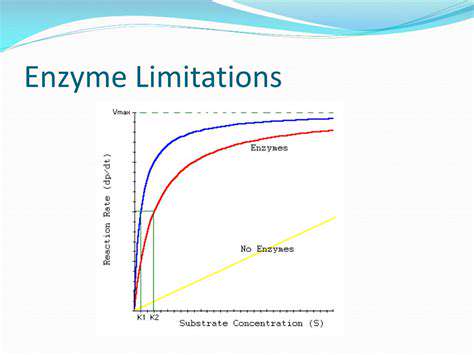
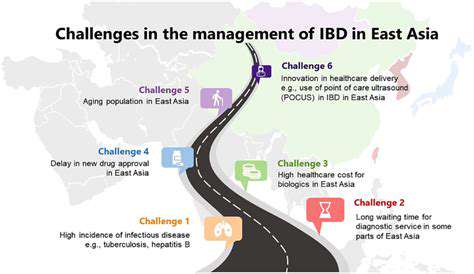
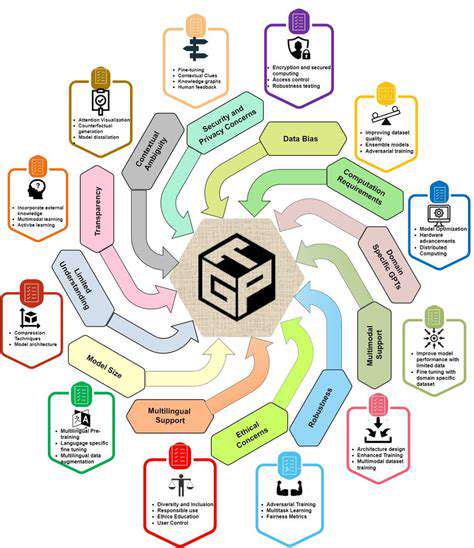
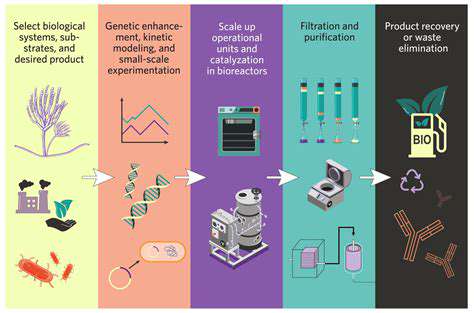
The pharmaceutical industry is undergoing a fundamental transformation as computational approaches supplement traditional laboratory techniques. Advanced algorithms now enable researchers to analyze molecular interactions at scales and speeds impossible through conventional methods. This computational power accelerates the identification of promising compounds while simultaneously reducing reliance on expensive and time-consuming experimental processes.
The most significant breakthroughs occur at the intersection of multiple disciplines, combining chemical informatics with biological insights and clinical data. This integrative approach allows researchers to evaluate potential drug candidates from multiple perspectives simultaneously, increasing the likelihood of identifying truly novel therapeutic approaches.
Tailoring Treatments to Individual Needs
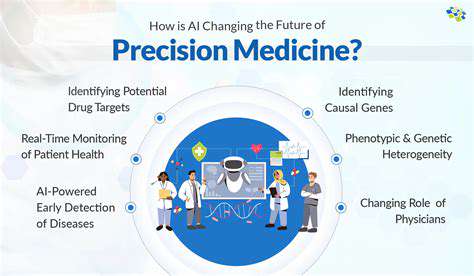
The concept of personalized medicine challenges traditional one-size-fits-all treatment paradigms. Contemporary approaches recognize that genetic variations, microbiome composition, and lifestyle factors collectively influence how individuals respond to medications. Sophisticated machine learning techniques can now integrate these diverse data sources to predict therapeutic outcomes with remarkable precision.
This individualized approach proves particularly valuable in oncology, where tumor genetics often determine treatment efficacy. By analyzing genetic markers, predictive models can suggest which therapies will likely succeed and which may cause adverse reactions, fundamentally changing patient care strategies.
Accelerated Drug Formulation and Development
Formulation science benefits significantly from computational modeling capabilities. Researchers can now simulate how different chemical structures will behave in biological systems, predicting absorption rates and metabolic pathways with increasing accuracy. These simulations guide the development of dosage forms optimized for specific patient populations or administration routes.
The ability to virtually test thousands of formulation variants before laboratory synthesis represents a major efficiency gain. This computational screening process dramatically reduces the number of physical experiments required, conserving both time and materials while increasing the probability of identifying optimal formulations.
Predicting Patient Responses to Drugs
Response prediction models integrate diverse patient data to forecast individual reactions to specific medications. These models consider genetic predispositions, concurrent medications, and physiological characteristics to generate personalized risk-benefit assessments. Clinicians can use these predictions to select the most appropriate therapies while minimizing potential side effects.
Emerging evidence suggests these predictive approaches may significantly improve medication adherence rates, as patients experience fewer adverse effects when prescribed medications compatible with their unique physiology. This represents an important secondary benefit beyond the primary therapeutic advantages.
Ethical Considerations and Future Implications
The rapid advancement of AI in medicine necessitates equally sophisticated ethical frameworks. Issues of data privacy become increasingly complex as models incorporate more personal health information. Additionally, ensuring equitable access to these advanced technologies presents both technical and policy challenges that the healthcare community must address.
Regulatory bodies face the difficult task of maintaining rigorous safety standards while allowing sufficient flexibility to accommodate innovative approaches. Striking this balance will be crucial as personalized medicine transitions from experimental applications to mainstream clinical practice across diverse healthcare systems.
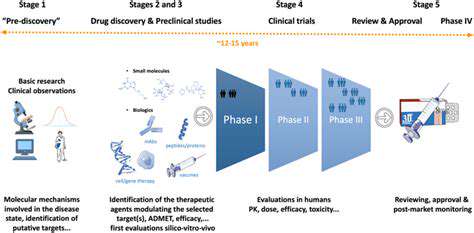
Accelerating the Drug Development Process with AI
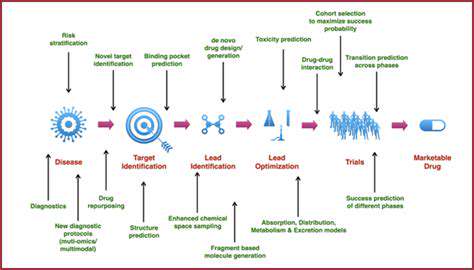
Streamlining the Pre-clinical Phase
The pre-clinical stage benefits tremendously from computational approaches that can prioritize the most promising candidates. Innovative screening technologies now allow researchers to evaluate thousands of compounds virtually, significantly reducing the number requiring physical testing. This computational triage conserves valuable laboratory resources while increasing the probability of identifying viable candidates.
Advanced modeling techniques also improve safety assessments by predicting potential toxicity profiles earlier in the development process. These predictions guide researchers toward compounds with favorable safety margins, reducing later-stage attrition due to unforeseen adverse effects.
Leveraging Computational Modeling
Molecular dynamics simulations have become indispensable tools for understanding drug-target interactions. These simulations provide atomic-level insights into binding mechanisms and residence times that inform structure-activity relationships. When combined with machine learning, these approaches can identify structural modifications likely to improve potency or selectivity.
The integration of quantum mechanical calculations with classical molecular dynamics represents a particularly promising frontier. This hybrid approach enables more accurate modeling of electronic interactions critical for understanding certain drug mechanisms.
Optimizing Clinical Trial Design
AI-driven trial design tools help researchers construct more efficient study protocols. These systems can analyze historical trial data to identify optimal patient populations, dosage regimens, and assessment schedules. The resulting designs often require fewer participants and shorter durations while maintaining statistical power.
Adaptive trial designs represent another innovation enabled by advanced analytics. These protocols allow modifications based on interim results without compromising study integrity, potentially accelerating development timelines while maintaining rigorous scientific standards.
Implementing Advanced Technologies
High-content screening systems now combine automated microscopy with image analysis algorithms to extract rich phenotypic data from cellular assays. These systems can detect subtle treatment effects that traditional assays might miss, providing deeper insights into compound mechanisms.
The combination of organ-on-a-chip technologies with AI analysis represents another transformative approach. These microphysiological systems better replicate human biology than conventional models, while machine learning extracts maximum information from the complex data they generate.
Enhancing Collaboration and Data Sharing
Cloud-based platforms facilitate secure data sharing among research institutions while maintaining appropriate privacy protections. These platforms often incorporate analytical tools that allow collaborators to derive insights without requiring extensive data science expertise.
Pre-competitive consortia have emerged as effective structures for addressing common challenges. By pooling resources and data on fundamental biological questions, these collaborations accelerate progress while reducing redundant efforts across the industry.
Focusing on Rare Diseases
AI approaches prove particularly valuable for rare disease research, where small patient populations traditionally made clinical studies challenging. Predictive models can extrapolate from limited data to identify promising therapeutic approaches that might otherwise be overlooked.
Natural language processing tools help researchers mine the scientific literature for overlooked connections, potentially identifying existing compounds that could be repurposed for rare conditions. This approach can dramatically shorten development timelines for these underserved patient populations.
AI-Powered Quality Control and Manufacturing Optimization
AI-Driven Inspection Systems
Modern quality control systems integrate multiple sensing modalities with advanced pattern recognition. These systems detect defects that might escape human inspectors while continuously learning from new examples to improve detection capabilities over time. The result is a dynamic quality assurance process that adapts to product variations and evolving standards.
Deep learning approaches have proven particularly effective for complex visual inspection tasks, such as identifying subtle material inconsistencies or microscopic structural flaws. These systems can process images at production-line speeds without compromising detection accuracy.
Predictive Maintenance for Optimized Operations
Equipment monitoring systems now incorporate sophisticated anomaly detection algorithms that identify subtle signs of impending failure. These systems analyze vibration patterns, thermal signatures, and performance metrics to forecast maintenance needs with increasing precision.
The integration of these predictive systems with inventory management creates a holistic approach to maintenance planning. Parts can be ordered automatically when needed, and maintenance scheduled during natural production lulls, minimizing disruption to operations while preventing catastrophic failures.
Optimized Production Scheduling and Resource Allocation
Advanced planning systems now consider hundreds of variables simultaneously to generate optimal production schedules. These systems balance equipment capabilities, material availability, labor constraints, and delivery commitments to maximize throughput while minimizing costs.
Reinforcement learning approaches show particular promise for dynamic rescheduling, allowing systems to continuously adjust plans in response to unexpected events like equipment breakdowns or rush orders while maintaining overall production goals.
Enhanced Supply Chain Visibility and Management
Modern supply chain platforms integrate data from multiple sources to provide comprehensive visibility. These systems can predict potential disruptions based on weather patterns, geopolitical events, or supplier performance trends, allowing proactive mitigation strategies.
The integration of blockchain technology with AI analytics creates tamper-proof audit trails while enabling smart contracts that automatically execute when predefined conditions are met. This combination enhances both transparency and efficiency in complex supply networks.
Improved Product Design and Development
Generative design tools powered by AI can explore vast design spaces to identify optimal solutions meeting multiple constraints. These systems propose innovative configurations that human designers might not consider, often achieving superior performance with less material usage.
Digital twin technology allows virtual testing of designs under realistic operating conditions, identifying potential issues before physical prototyping. This approach reduces development cycles while improving final product quality and reliability.