Rigorous Methodology: Ensuring Scientific Validity
Defining Rigorous Methodology
Methodological rigor constitutes the steel framework supporting credible clinical research. This comprehensive approach governs every study aspect from initial design to final analysis, systematically eliminating potential biases and confounding factors. A meticulously crafted protocol serves as the study blueprint, detailing participant criteria, intervention parameters, data collection standards, and analytical strategies with precision. This level of documentation ensures complete transparency and reproducibility - hallmarks of trustworthy science.
Bias mitigation requires constant vigilance across all trial phases. Strategic participant recruitment minimizes selection bias, standardized protocols reduce information bias, and blinding procedures counteract performance bias. Collectively, these safeguards preserve study integrity while enhancing result reliability.
Participant Selection and Randomization
Study validity hinges on assembling participant cohorts that accurately mirror target patient populations. Carefully constructed inclusion/exclusion criteria act as filters, ensuring enrolled individuals properly represent those who might eventually use the treatment. These criteria demand clear justification and strict enforcement to maintain population homogeneity and minimize confounding variables.
Randomization serves as the great equalizer in clinical research. This process distributes participants randomly between treatment and control groups, neutralizing selection bias and ensuring group comparability. Advanced techniques like stratified randomization further enhance balance across key demographic or clinical characteristics, strengthening the study's evidentiary value.
Blinding and Placebo Control
The blinding technique represents a powerful tool for maintaining objectivity in clinical assessments. Whether single-blind (participants unaware) or double-blind (both participants and investigators unaware), this approach prevents conscious or subconscious biases from coloring outcome evaluations. Particularly crucial for subjective endpoints like pain assessment, blinding helps isolate the true treatment effect from psychological influences.
Placebo controls establish the essential baseline for treatment evaluation. These inert interventions, indistinguishable from active treatments, enable researchers to distinguish pharmacological effects from placebo responses. While ethically complex in certain contexts, properly implemented placebo controls significantly enhance study validity when alternative designs prove inadequate.
Data Collection and Management
High-quality data forms the lifeblood of credible research. Standardized collection instruments and protocols ensure consistency across study sites and evaluators. Validated assessment tools and precisely defined operational criteria minimize measurement variability, while comprehensive documentation practices create an auditable trail from raw data to published results.
Robust data management systems form the infrastructure supporting research integrity. These incorporate rigorous quality control measures, secure storage solutions, and redundant backup protocols. Together, they safeguard against data corruption or loss while facilitating efficient analysis and regulatory review.
Statistical Analysis and Interpretation
Statistical methodology converts data into evidence through appropriate analytical techniques. Selecting the right tests requires careful consideration of data types, distribution characteristics, and study objectives. Qualified statisticians must oversee this process, applying methods that properly account for study design elements like clustering or repeated measures.
Interpretation demands equal rigor, requiring researchers to contextualize findings within study limitations and potential confounders. Transparent reporting of analytical methods, assumptions, and uncertainties allows the scientific community to properly evaluate evidence strength. This intellectual honesty strengthens research credibility and guides appropriate clinical application.
Ethical Considerations and Regulatory Compliance
Ethical principles form the moral foundation of clinical research. Participant protections extend beyond informed consent to encompass ongoing safety monitoring, equitable subject selection, and fair benefit-risk ratios. Institutional Review Boards provide essential independent oversight, ensuring studies meet ethical benchmarks before commencement and throughout execution.
Regulatory compliance provides the structural framework for ethical research. Good Clinical Practice guidelines establish universal standards for trial conduct, data integrity, and participant protections. Adherence to these standards ensures research quality while maintaining public trust in medical science.
The Future of Clinical Trials: Innovation and Advancements
Decentralized Clinical Trials: Expanding Access and Efficiency
Geographical barriers traditionally limited clinical trial participation, but decentralized models are dismantling these obstacles. By leveraging digital health technologies, researchers can now engage participants regardless of location, dramatically expanding study diversity and representation. Wearable biosensors, mobile health apps, and telemedicine platforms facilitate remote data collection while reducing participant burden. This paradigm shift not only enhances convenience but also potentially accelerates enrollment and reduces operational costs.
The efficiency gains from decentralized approaches could significantly shorten development timelines for new therapies. Reduced site visit requirements and automated data capture streamline processes while maintaining rigorous standards, potentially bringing treatments to market faster without compromising scientific integrity.
AI-Powered Data Analysis: Accelerating Insights
Artificial intelligence is revolutionizing clinical research by uncovering hidden patterns in complex datasets. Machine learning algorithms can process vast amounts of multimodal data - from genomic profiles to continuous physiological monitoring - identifying subtle treatment effects and safety signals that might elude conventional analysis. These capabilities enable more nuanced understanding of treatment responses across diverse patient subgroups.
Predictive analytics represent another AI frontier, potentially forecasting individual treatment responses based on baseline characteristics. This capability could optimize trial designs by identifying likely responders earlier, making studies more efficient while personalizing patient care.
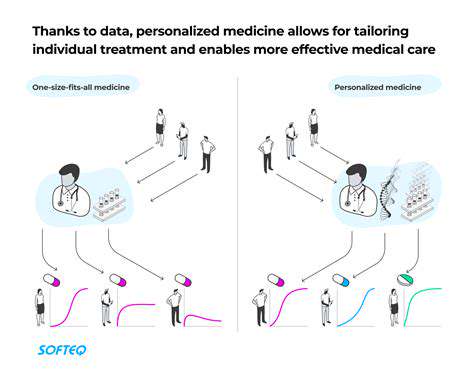
Personalized Medicine: Tailoring Treatments to Individual Needs
The era of one-size-fits-all medicine is giving way to precision approaches that account for individual variability. Clinical trials increasingly incorporate biomarker strategies to match specific treatments with patients most likely to benefit. This paradigm shift requires novel trial designs like basket trials (grouping by molecular markers rather than tumor type) or umbrella trials (testing multiple targeted therapies within a single disease).
Implementing precision medicine trials demands close collaboration between clinicians, geneticists, and bioinformaticians. The resulting studies generate insights that propel drug development toward increasingly targeted, effective therapies with minimized side effects.
Advanced Imaging Techniques: Enhanced Diagnostic Capabilities
Modern imaging technologies provide unprecedented windows into disease processes and treatment effects. High-resolution modalities like functional MRI and PET scans reveal physiological changes at molecular levels, while AI-enhanced image analysis detects subtle patterns invisible to human observers. These capabilities enable more precise disease staging, treatment response assessment, and safety monitoring.
Standardized imaging protocols across study sites enhance data comparability, while quantitative analysis techniques reduce subjective interpretation. Together, these advances produce more objective, reproducible endpoints that strengthen clinical evidence.
Ethical Considerations in Clinical Trial Design
Innovative trial designs must balance scientific ambition with ethical responsibility. As studies become more complex - incorporating genetic testing, digital monitoring, or artificial intelligence - informed consent processes must similarly evolve. Participants deserve clear explanations of novel technologies and their implications, ensuring comprehension matches protocol complexity.
Ethical oversight must keep pace with technological advancement. Review boards require expertise in emerging technologies to properly evaluate risks and benefits. Continuous ethics education ensures all stakeholders maintain awareness of evolving best practices in this dynamic landscape.
Data Security and Privacy: Protecting Patient Information
The digital transformation of clinical research amplifies data security imperatives. With studies increasingly relying on electronic health records, wearable devices, and mobile apps, robust cybersecurity measures become non-negotiable. Encryption standards, access controls, and blockchain technologies help safeguard sensitive health information against evolving threats.
Privacy protections must extend beyond compliance checkboxes to embody ethical commitments. Transparent data use policies, granular participant controls over information sharing, and breach notification protocols demonstrate respect for participant autonomy while maintaining trust in clinical research ecosystems.