Automated Liquid Handling Systems: A Comprehensive Overview
Automated liquid handling systems have become indispensable in modern laboratories, revolutionizing workflows in drug discovery, biotechnology, and chemical analysis. By automating liquid transfers, these systems minimize human intervention while enhancing precision. What makes them truly remarkable is their capacity to execute repetitive tasks with unparalleled accuracy, ensuring experimental consistency that manual methods struggle to match. This technological advancement frees researchers from tedious manual pipetting, allowing them to dedicate more time to critical data interpretation.
Modern systems combine robotic arms with specialized pipetting tools and intelligent software. This integration enables precise control over every aspect of liquid transfer – from volume measurement to dispensing speed and target location. Such precision becomes particularly valuable in high-throughput environments where processing hundreds of samples accurately and quickly is non-negotiable. The software's programmable nature allows for customization of protocols to meet specific experimental requirements.
Key Benefits of Automated Liquid Handling
The primary advantage lies in error reduction. Even skilled technicians can introduce variability through manual pipetting, but automated systems virtually eliminate this issue. This consistency proves crucial in research environments where reproducible results form the foundation of scientific progress. The impact extends beyond accuracy – automation dramatically increases processing capacity, enabling laboratories to handle larger sample volumes without compromising quality.
Time efficiency represents another significant benefit. Automated platforms can complete in hours what might take days manually, particularly valuable in time-sensitive research or diagnostic applications. By accelerating sample preparation, these systems allow scientists to focus on more complex analytical challenges. Additionally, they enhance laboratory safety by minimizing direct contact with potentially hazardous substances, creating a safer work environment.
Types of Automated Liquid Handling Systems
The market offers diverse systems tailored to specific applications, ranging from basic liquid transfer units to sophisticated platforms handling complex protocols. Some specialize in microplate processing, making them ideal for high-volume screening, while others accommodate various container formats. The selection process should consider multiple factors including working volume, throughput requirements, and protocol complexity. Choosing the right system involves balancing current needs with future scalability to maximize long-term laboratory efficiency.
Applications in Different Fields
These systems find applications across multiple scientific disciplines. Pharmaceutical researchers utilize them extensively in drug discovery pipelines, particularly in screening potential therapeutic compounds. In biotech applications, they're instrumental in cell culture maintenance and genetic engineering workflows. Industrial chemistry laboratories employ them for precise reagent preparation and quality control procedures. Academic institutions benefit from their precision in diverse research projects, from molecular biology to environmental science.
High-Throughput Screening and Data Analysis: Accelerating Discovery
High-Throughput Screening Strategies
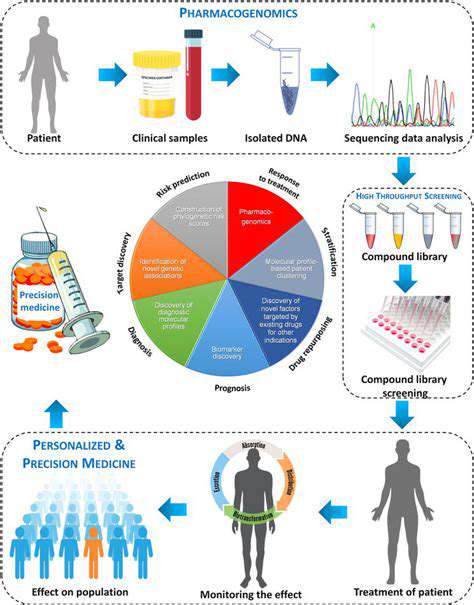
High-throughput screening (HTS) has transformed how researchers identify bioactive compounds. This approach enables rapid evaluation of thousands of compounds, dramatically accelerating the identification of promising candidates. Various screening strategies exist, each designed to answer specific research questions, whether identifying enzyme inhibitors or detecting molecular interactions.
Data Acquisition and Management
Effective HTS depends on robust data collection systems. Modern instruments generate vast amounts of data that require careful organization. Implementing proper data management protocols ensures information remains accessible for analysis while maintaining integrity. This includes using specialized databases that can handle the volume and complexity of screening data while facilitating easy retrieval.
Data Analysis Techniques
Analyzing HTS results requires sophisticated approaches to distinguish meaningful patterns from background noise. Statistical methods form the foundation, but increasingly, machine learning algorithms provide additional analytical power. These advanced techniques help researchers identify subtle but significant correlations that might otherwise go unnoticed.
Validation and Interpretation of Results
Initial screening hits require rigorous validation to confirm biological activity. Careful experimental design during this phase helps avoid false positives and ensures reliable conclusions. Researchers typically employ orthogonal assays to verify initial findings before committing resources to further development.
Quality Control in HTS
Maintaining quality throughout the screening process is paramount. This involves careful monitoring of reagent quality, instrument performance, and procedural consistency. Implementing comprehensive quality control measures ensures the generation of reliable, reproducible data that forms a solid foundation for decision-making.
Automation in DNA Synthesis and Sequencing: Facilitating Design and Validation
Improving Design Efficiency
Automation has revolutionized DNA sequence design through specialized software that optimizes oligonucleotide sequences. These tools perform complex calculations predicting molecular behavior, allowing researchers to focus on experimental design rather than manual sequence optimization.
Streamlining Synthesis Procedures
Automated synthesizers produce DNA with exceptional consistency, minimizing synthesis errors that plagued earlier manual methods. Integrated purification systems further enhance output quality, delivering ready-to-use DNA for downstream applications.
Enhancing Sequencing Accuracy and Speed
Modern sequencing platforms leverage automation to process samples rapidly while maintaining high accuracy. This capability has enabled projects that would have been impractical with manual methods, from large-scale genomic studies to personalized medicine applications.
Validating Results through Automation
Automated quality checks during synthesis and sequencing provide immediate feedback on product quality. This real-time monitoring allows for early detection of potential issues, preventing wasted resources on compromised samples.
Facilitating Data Analysis and Interpretation
Advanced bioinformatics tools automate the interpretation of sequencing data, identifying patterns and variations efficiently. This automation enables researchers to extract meaningful biological insights from complex datasets more rapidly than ever before.
The Future of Automation in Synthetic Biology Labs
The Rise of AI-Powered Synthesis
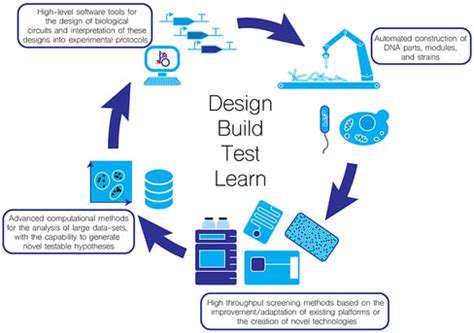
Artificial intelligence is revolutionizing synthetic biology by analyzing chemical reaction patterns and predicting outcomes with unprecedented accuracy. This technology enables the design of novel synthetic pathways that were previously unimaginable.
Automation in Laboratory Settings
Modern automated systems handle increasingly complex procedures with minimal human intervention. This integration of robotics into laboratory workflows significantly enhances both productivity and data reliability.
Scalability and Reproducibility
Automation provides the consistency required to scale processes from laboratory to industrial production. Precise control over reaction parameters ensures product quality remains constant across production batches.
Enhanced Safety and Efficiency
Automated systems minimize human exposure to hazardous materials while improving workflow efficiency. This dual benefit makes automation particularly valuable in handling dangerous or sensitive materials.
Economic Implications
The economic impact of laboratory automation continues to grow as systems become more sophisticated. These technologies are creating new opportunities for innovation while reducing production costs across multiple industries.
Ethical Considerations
As automation advances, it's crucial to consider its broader societal implications. Responsible development of these technologies requires careful consideration of accessibility and workforce impacts.