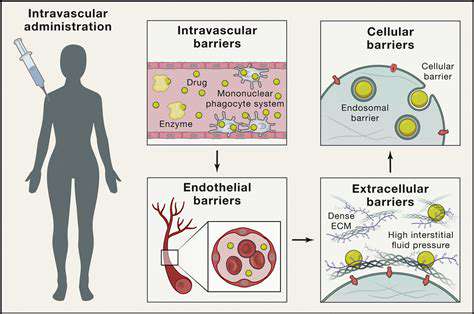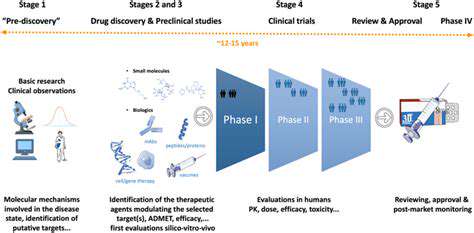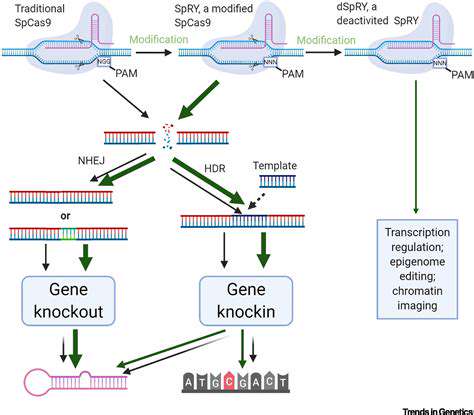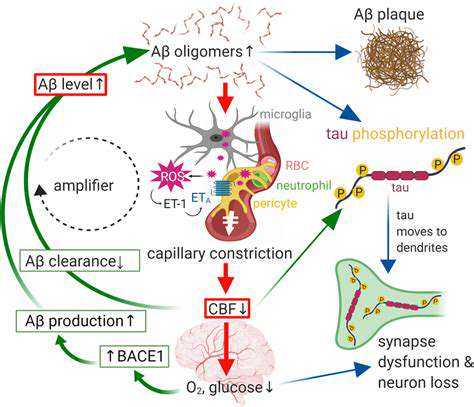
Addressing Challenges in Clinical Trials and Regulatory Processes
Improving Participant Recruitment and Retention
A crucial aspect of successful clinical trials is ensuring a diverse and representative pool of participants. Recruitment strategies must be robust and tailored to specific patient populations, encompassing various communication channels and outreach efforts. This includes leveraging community partnerships, social media campaigns, and targeted advertising to reach potential participants. Furthermore, providing clear and concise information about the trial's purpose, procedures, and potential benefits is essential to fostering trust and encouraging enrollment. Maintaining participant engagement and retention throughout the trial is equally vital. Well-defined communication plans, regular check-ins, and addressing any concerns promptly are key factors for minimizing attrition rates.
Addressing Ethical Considerations in Clinical Trials
Clinical trials must adhere to the highest ethical standards, ensuring the safety and well-being of all participants. This involves rigorous ethical review by independent committees, transparent consent procedures, and ongoing monitoring of participant data. Maintaining confidentiality and protecting sensitive participant information is paramount. Furthermore, the potential risks and benefits of the treatment under investigation must be carefully weighed and communicated to participants. Researchers must prioritize participant autonomy and respect their right to withdraw from the trial at any point.
Navigating Complex Regulatory Environments
Navigating the complex regulatory landscape is a significant challenge for drug developers. Compliance with regulations related to study design, data management, and reporting is crucial. Understanding and adhering to the specific requirements of different regulatory bodies across various countries and regions is essential. This involves meticulous documentation, rigorous data validation procedures, and ensuring compliance with Good Clinical Practice (GCP) guidelines. The intricacies of regulatory pathways can be time-consuming, requiring careful planning and expert guidance throughout the process.
Optimizing Trial Design for Efficiency and Accuracy
Efficient and accurate trial design is critical for minimizing costs and timelines. Careful consideration of sample size calculations, data collection methods, and statistical analysis plans are essential. Utilizing innovative technologies, such as electronic data capture systems and remote monitoring tools, can significantly enhance efficiency and accuracy. Ensuring data quality throughout the entire trial process is crucial for reliable results and robust conclusions. This requires robust data management systems and rigorous quality control measures.
Managing Financial Resources Effectively
Clinical trials require significant financial resources to cover various aspects, including participant compensation, site facilities, data analysis, and regulatory compliance. Developing a detailed budget, securing necessary funding, and managing expenses effectively are crucial for project feasibility. Strategic cost-saving measures should be explored without compromising the quality and integrity of the trial. Careful planning and proactive financial management are essential for successful project completion.
Ensuring Data Integrity and Analysis
Maintaining data integrity and conducting rigorous statistical analysis are vital components of clinical trials. Developing clear data collection protocols, implementing robust data management systems, and validating data entries are all essential. The analysis of collected data should be conducted by qualified statisticians using appropriate methodologies. This ensures the accuracy and reliability of the results, which are crucial for drawing valid conclusions. Furthermore, ensuring data security and privacy is of utmost importance.
Collaboration and Communication Throughout the Process
Effective collaboration and communication among various stakeholders, including researchers, regulatory bodies, sponsors, and participants, are crucial for successful clinical trials. Open communication channels, clear roles and responsibilities, and regular progress updates are necessary for a smooth process. This includes strong communication strategies to ensure transparency and address concerns promptly. Building strong relationships and fostering trust among all parties involved is essential for navigating the complexities of clinical research.
Cultivating International Collaboration and Public-Private Partnerships

Fostering Global Partnerships
Cultivating international collaborations is crucial for advancing knowledge, innovation, and problem-solving on a global scale. These partnerships can unlock a wider range of perspectives and expertise, leading to more robust and impactful solutions. By bridging cultural divides and fostering mutual understanding, we can address complex challenges more effectively. International collaboration is a powerful tool for driving progress in various fields, including scientific research, technological advancements, and social development initiatives.
International collaborations often result in the sharing of resources and expertise, which can accelerate the pace of progress in any given field. This exchange of knowledge and resources can lead to breakthroughs that might otherwise take much longer or even remain elusive.
Leveraging Diverse Perspectives
International collaborations offer a rich tapestry of diverse perspectives, experiences, and approaches. By engaging with individuals and organizations from different cultural backgrounds, we can gain valuable insights and broaden our understanding of the world. This diverse input can lead to innovative ideas and more comprehensive solutions to global problems.
Understanding the different cultural norms and values is crucial for successful international partnerships. This understanding is essential for effective communication and collaboration across national boundaries. By acknowledging and respecting these differences, we can foster a more inclusive and productive environment for collaboration.
Expanding Research Horizons
International collaborations often lead to the expansion of research horizons. Researchers from different countries can pool their knowledge and resources to address complex scientific questions that may be too challenging for a single institution or nation to tackle alone. This collaborative approach can accelerate the pace of scientific discovery and lead to advancements in various fields.
International collaborations can facilitate access to cutting-edge technologies and resources that may not be readily available in a single country. This access can open up new possibilities for research and development, leading to significant breakthroughs in the future.
Enhancing Technological Advancement
International collaborations play a vital role in driving technological advancement. By combining resources, expertise, and knowledge from various nations, we can accelerate the development and implementation of new technologies. This collaborative approach can lead to the creation of innovative solutions to pressing global challenges.
International collaborations can foster the development of new technologies through the sharing of ideas, knowledge, and resources. This shared approach to innovation can create a more interconnected and progressive global landscape.
Promoting Cultural Exchange and Understanding
International collaborations serve as powerful platforms for promoting cultural exchange and understanding. By bringing together individuals from different cultural backgrounds, we can foster mutual respect and appreciation for diverse perspectives. This exchange of ideas and experiences can help break down stereotypes and prejudices, paving the way for a more peaceful and harmonious world.
Through international collaborations, individuals gain valuable insights into different cultures and ways of life. This exposure to diverse cultures can lead to greater empathy and understanding, promoting tolerance and cooperation on a global scale.