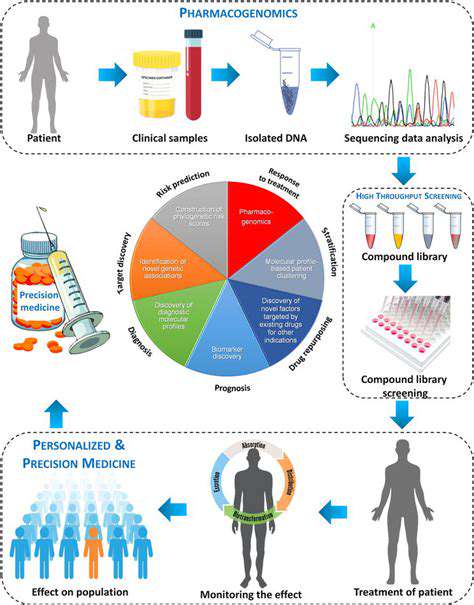
Precision medicine represents a paradigm shift in healthcare, moving away from generalized treatments toward strategies customized to each patient's genetic profile, lifestyle, and environment. This tailored methodology enhances treatment efficacy while reducing harmful side effects by accounting for individual variability. Rather than applying uniform solutions, it embraces the intricate diversity of human biology.
Through analysis of unique genetic markers and other contributing elements, precision medicine enables earlier disease detection, more accurate risk assessment, and therapies with higher success rates and fewer complications. Its impact is particularly profound in oncology, where tumor genetics significantly influence disease trajectories.
Genomic Sequencing and its Impact
Modern genomic sequencing technologies have revolutionized medical diagnostics by allowing comprehensive DNA analysis. These tools identify genetic variations that may predispose individuals to certain conditions or affect treatment responses. Such detailed genomic profiles empower clinicians to predict health risks and customize interventions with unprecedented precision.
Pharmacogenomics: Tailoring Medications
As a cornerstone of precision medicine, pharmacogenomics examines how genetic differences influence drug metabolism and effectiveness. This knowledge enables healthcare providers to prescribe optimal medications and dosages, minimizing adverse reactions while maximizing therapeutic benefits. The personalized medication approach significantly enhances patient outcomes and quality of life.
Pharmacogenomic testing dramatically reduces medication-related complications by identifying drugs most compatible with a patient's genetic makeup, ensuring safer and more effective treatment regimens.
Personalized Cancer Treatment Strategies
Oncology has been transformed by precision medicine's ability to develop therapies targeting specific tumor mutations. By analyzing a cancer's genetic signature, oncologists can select treatments that precisely attack malignant cells while sparing healthy tissue. This approach has improved survival rates and reduced treatment side effects for many cancer patients.
Challenges and Future Directions
While promising, precision medicine faces obstacles including high costs and unequal access to advanced diagnostics and treatments. Widespread implementation requires addressing these barriers while integrating precision tools into standard medical practice. Future success depends on making these technologies more affordable and accessible across diverse populations.
Ethical Considerations in Precision Medicine
The rise of precision medicine brings important ethical questions regarding genetic data privacy and potential discrimination. Protecting sensitive health information while ensuring equitable access to personalized treatments requires thoughtful policies and transparent communication. Maintaining public trust necessitates clear guidelines on data usage and strong safeguards against genetic discrimination.
The Importance of Clinical Trial Design and Collaboration
Defining the Scope of the Rare Disease
Designing clinical trials for rare diseases demands meticulous population definition, considering both the condition's genetic and phenotypic characteristics and potential variations among patients. Precise participant selection ensures study results remain clinically relevant and scientifically valid.
Comprehensive inclusion criteria should account for disease severity across its spectrum, from mild to advanced cases. This thorough approach facilitates accurate assessment of treatment effects at different disease stages, establishing clear therapeutic correlations. Proper patient selection forms the foundation for subsequent trial phases.
Importance of Patient Recruitment and Retention
Rare disease trials face unique recruitment challenges due to small patient pools. Effective strategies involve partnering with advocacy groups, implementing genetic screening initiatives, and engaging specialist physician networks. Ensuring demographic diversity across age, gender, and disease severity enhances study validity.
Retention strategies must address logistical barriers through clear communication, participant education, and support services. Minimizing geographic and access-related obstacles helps maintain study cohorts and data integrity throughout trial durations.
Developing a Robust and Ethical Study Protocol
Well-structured protocols are essential for rare disease research success. These documents should clearly define study objectives, methodologies, inclusion/exclusion criteria, interventions, and analytical approaches while addressing condition-specific challenges.
Ethical considerations remain paramount, requiring rigorous protections for participant safety, informed consent procedures, data confidentiality, and access to medical support. These safeguards maintain research integrity while protecting vulnerable populations.
Utilizing Innovative Data Collection Methods
Advanced data gathering techniques, including remote monitoring and digital health tools, can enhance rare disease studies by providing comprehensive real-world data while minimizing patient burden. These innovations help overcome traditional research limitations in sparse populations.
Collaboration between Researchers and Stakeholders
Successful rare disease research requires strong partnerships across academia, clinical practice, patient advocacy, and regulatory bodies. Such collaborations pool expertise and resources, accelerating therapeutic development while ensuring studies address real patient needs.
Multidisciplinary cooperation facilitates knowledge sharing, enabling researchers to overcome scientific and logistical challenges more effectively. These joint efforts ultimately improve treatment options and care standards for rare disease patients.
Addressing Challenges Specific to Rare Diseases
Rare disease trials must overcome obstacles including small sample sizes, recruitment difficulties, and specialized care requirements. Innovative solutions involve leveraging patient registries, adopting adaptive trial designs, and fostering international research networks.
Strategic partnerships with advocacy organizations and specialized clinical centers help address these challenges, advancing understanding and treatment of rare conditions through collaborative science.
Funding and Resources: Addressing the Financial Barriers
Understanding the Financial Landscape of Rare Disease Drug Development
The economics of rare disease drug development present unique challenges, as limited patient populations reduce commercial incentives for pharmaceutical investment. Funding typically combines public grants, private philanthropy, and foundation support, requiring sophisticated proposal development and stakeholder engagement.
Government Funding Initiatives and Programs
Public sector programs play a vital role in rare disease research, supporting basic science, diagnostic development, and clinical trials. These initiatives prioritize high-impact projects with potential to transform patient care, often complemented by tax incentives to stimulate private investment.
Private Sector Investment and Venture Capital
While growing, private investment in rare diseases remains cautious due to financial risks. Innovative financing models and careful therapeutic target selection can attract capital by demonstrating viable pathways to clinical and commercial success.
Philanthropic Contributions and Foundations
Disease-specific foundations provide critical funding for research and patient services, often driven by personal connections to rare conditions. Their support bridges gaps in traditional funding streams, enabling high-risk/high-reward investigations.
The Role of Patient Advocacy Groups and Organizations
Advocacy organizations amplify patient voices in research prioritization and policy discussions. By connecting stakeholders and raising awareness, they help direct resources toward the most pressing unmet medical needs in rare diseases.
Accessing and Utilizing Existing Resources
Researchers must strategically leverage available tools including clinical databases, collaborative networks, and shared research infrastructure. Identifying synergies across studies maximizes resource efficiency and accelerates therapeutic development.
The Importance of International Collaboration
Global cooperation is essential for rare disease research, enabling data sharing, coordinated trials, and pooled expertise across borders. These international partnerships enhance study power and facilitate treatment development for geographically dispersed patient populations.