Revolutionizing Alzheimer's Research Through CRISPR-Cas9 Technology
The CRISPR-Cas9 Breakthrough in Neurological Studies
This groundbreaking gene-editing system has transformed our approach to studying neurodegenerative disorders. Scientists can now pinpoint and alter specific genetic sequences with remarkable accuracy, opening new avenues for understanding Alzheimer's complex genetic architecture. This precision tool enables researchers to dissect the intricate web of genetic interactions that underlie disease progression, paving the way for targeted interventions.
Addressing Amyloid-β Pathology
The technology's application in studying amyloid-β plaque formation represents a significant advancement. These neural deposits, characteristic of Alzheimer's pathology, correlate strongly with cognitive deterioration and neuronal damage. Investigators are employing CRISPR-Cas9 to identify key genetic components involved in amyloid-β synthesis and clearance mechanisms.
The ability to precisely regulate amyloid-β concentrations using this technology could revolutionize therapeutic approaches by targeting core disease mechanisms.
Intervening in Tau Protein Dynamics
Tau protein abnormalities represent another critical area of investigation. The formation of neurofibrillary tangles from misfolded tau proteins constitutes a fundamental pathological process in Alzheimer's progression. Current research focuses on identifying genetic targets that influence tau phosphorylation and aggregation patterns.
Decoding Genetic Susceptibility
The multifactorial nature of Alzheimer's involves numerous genetic components. CRISPR-Cas9 facilitates detailed examination of these risk factors, allowing researchers to determine how specific genetic variations influence disease vulnerability. This granular understanding is essential for developing customized prevention protocols and treatment plans.
Innovative Treatment Development
Therapeutic applications extend beyond basic research, with potential for direct genetic intervention. Scientists are exploring the feasibility of correcting pathological mutations in affected neural cells, representing a potential paradigm shift in treatment methodology. The primary obstacle remains developing reliable delivery systems for genetic material into cerebral tissue.
Modulating Neuroinflammatory Processes
Chronic inflammation significantly contributes to Alzheimer's progression. CRISPR-Cas9 enables researchers to investigate genetic regulation of inflammatory pathways, including possibilities for modifying these responses to potentially slow disease advancement. This research direction may yield interventions targeting the inflammatory components of neurodegeneration.
Ethical Dimensions and Future Prospects
While the therapeutic potential is immense, ethical considerations regarding genetic modification demand careful deliberation. Key concerns include unintended genetic alterations, safety protocols, and equitable treatment access. Future development must prioritize enhanced delivery mechanisms, improved editing accuracy, and comprehensive risk assessment to ensure responsible implementation. The ultimate success of this technology depends on balancing innovation with ethical responsibility.
Combatting Protein Accumulation in Alzheimer's Pathology
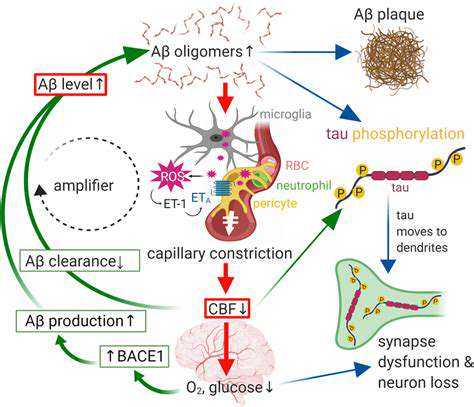
Amyloid-β Plaque Intervention
Amyloid-β deposition disrupts neural communication and synaptic function. Substantial evidence links these deposits to progressive cognitive impairment in affected individuals. Current research focuses on understanding the molecular mechanisms governing Aβ formation and aggregation, which is critical for developing effective countermeasures.
Multiple therapeutic strategies are under investigation, including enzymatic inhibition of Aβ production and disruption of aggregation processes. While promising, significant hurdles remain in adapting these approaches for clinical use, particularly regarding safety profiles and treatment efficacy.
Tauopathy Management
Under normal conditions, tau proteins stabilize neuronal microtubules. In Alzheimer's disease, abnormal phosphorylation leads to tangle formation that impairs cellular transport systems, resulting in neuronal dysfunction and death.
The correlation between tau pathology and cognitive symptoms underscores the importance of understanding phosphorylation mechanisms. Identifying molecular targets to prevent or reverse tau abnormalities represents a crucial therapeutic objective.
Pharmaceutical Development
Numerous drug candidates targeting Aβ and tau pathways are in various stages of development. The challenge lies in creating compounds that effectively modulate these proteins while maintaining acceptable safety margins.
Preliminary clinical trial data shows some encouraging results, though extensive research is still required to establish optimal treatment protocols and long-term outcomes.
Immunotherapeutic Options
Antibody-based approaches, including both passive and active immunization strategies, show potential for clearing pathological protein accumulations. These methods harness the immune system to target and eliminate abnormal protein deposits.
Genetic Intervention Approaches
Gene therapy offers the possibility of fundamentally altering the production of pathological proteins. By modifying gene expression patterns, this approach could potentially prevent protein accumulation at its source.
Preventative Lifestyle Factors
Non-pharmacological interventions play a significant role in risk reduction and disease management. A comprehensive approach incorporating nutritional optimization, physical activity, and cognitive engagement can significantly influence brain health outcomes. While not curative, these measures may delay symptom onset and slow disease progression.
Obstacles and Future Perspectives
Precision and Delivery Challenges
The primary technical challenges involve the specificity of genetic modifications and effective delivery to target tissues. While current technologies demonstrate impressive accuracy, the possibility of unintended genetic alterations remains a significant concern. This necessitates ongoing refinement of targeting mechanisms and rigorous validation protocols.
The blood-brain barrier presents additional delivery complications, requiring innovative solutions such as viral vectors or nanoparticle carriers. The success of clinical applications depends heavily on developing reliable delivery systems that can safely transport genetic material to affected brain regions.
Ethical and Societal Implications
The potential for heritable genetic modifications raises profound ethical questions that require thorough examination. Public engagement and interdisciplinary ethical review processes are essential for establishing appropriate guidelines and ensuring responsible development of these technologies.
The long-term consequences of germline editing necessitate careful consideration and robust regulatory frameworks. A comprehensive dialogue involving diverse stakeholders is crucial for navigating these complex issues.
Enhancing Specificity
Ongoing research focuses on improving the precision of gene editing tools to minimize off-target effects. This involves developing novel editing enzymes and optimizing existing systems to achieve greater accuracy. Such advancements are critical for ensuring treatment safety and efficacy.
Treatment Accessibility
The economic aspects of gene therapy development present significant barriers to widespread implementation. Developing cost-effective production methods and equitable distribution models will be essential for maximizing the societal benefits of these advanced treatments.
Combinatorial Therapeutic Approaches
Integrating gene editing with conventional treatments may offer synergistic benefits for managing Alzheimer's complex pathology. The combination of genetic interventions with pharmacological and lifestyle approaches could provide more comprehensive therapeutic strategies. This integrated approach may yield superior clinical outcomes by addressing multiple disease pathways simultaneously.