While CRISPR-Cas9 dominates the headlines, other gene-editing technologies are also emerging as promising avenues for cancer treatment. Zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs), though less precise than CRISPR in some instances, offer alternative pathways for gene editing and have already shown potential in preclinical studies. Understanding the strengths and weaknesses of each technology is crucial for selecting the most appropriate tool for a specific cancer type and individual patient.
The exploration of alternative gene editing tools is vital for expanding the therapeutic arsenal against cancer. Different technologies may have unique advantages in targeting specific genes or cell types, leading to more precise and effective treatment strategies. Ongoing research aims to refine these techniques and potentially combine them with other therapeutic approaches to create comprehensive and personalized cancer treatment plans.
Personalized Medicine and the Future of Gene Editing
The ability to tailor gene editing strategies to individual patients represents a paradigm shift in cancer treatment. By analyzing a patient's specific genetic makeup, doctors can identify vulnerabilities in the cancer cells and design gene editing therapies targeted to those vulnerabilities. This personalized approach holds the promise of increasing treatment efficacy and minimizing side effects.
The integration of gene editing into personalized medicine approaches promises to be a transformative force in the fight against cancer. Future research will likely focus on developing sophisticated diagnostic tools to identify ideal gene editing targets in individual patients and optimizing delivery methods to maximize the effectiveness of the treatment. This personalized approach has the potential to revolutionize cancer care and lead to improved outcomes for patients.
Ethical Considerations and Regulatory Challenges
The rapid advancement of gene editing technologies necessitates careful consideration of the ethical implications and regulatory challenges associated with their clinical application. Issues surrounding safety, off-target effects, and equitable access to these potentially life-saving therapies need to be addressed. Establishing clear guidelines and protocols for clinical trials is paramount to ensuring responsible and ethical development and deployment of these powerful tools.
Stringent regulatory frameworks are essential to manage the risks and ensure the responsible use of gene editing technologies in cancer treatment. Transparency and open dialogue among researchers, clinicians, ethicists, and policymakers are critical for navigating the complex ethical and societal implications of this transformative technology. Careful consideration of long-term impacts and potential unintended consequences is crucial to ensure that gene editing therapies are used in a safe and responsible manner.
Clinical Trials and the Path to Implementation
The journey from laboratory research to clinical application for gene editing in cancer treatment requires rigorous clinical trials to assess safety and efficacy. These trials will need to carefully evaluate the impact of gene editing therapies on various cancer types and patient populations. Developing robust and reliable methods for monitoring treatment response and potential side effects is essential for successful clinical translation.
The translation of gene editing technologies into clinical practice is a complex process that requires meticulous planning, rigorous testing, and careful consideration of potential risks and benefits. Clinical trials are essential to validate the safety and efficacy of these therapies in real-world settings, ensuring their responsible implementation and maximizing their potential to improve patient outcomes. Building trust and transparency through open communication between researchers, clinicians, and patients is crucial in this process.
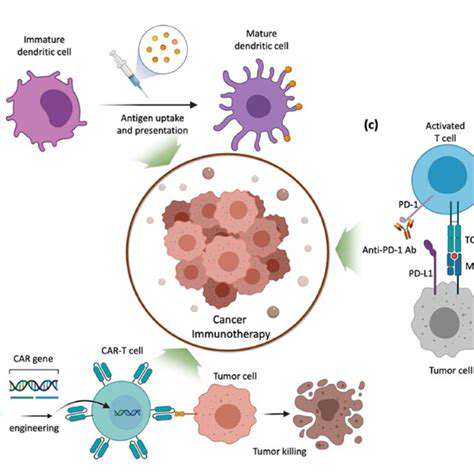
Beyond CAR-T: Targeting Cancer Cells Directly with Gene Editing
Harnessing the Power of CRISPR-Cas9
CRISPR-Cas9, a revolutionary gene-editing technology, offers unprecedented precision in targeting and modifying specific DNA sequences within cells. This powerful tool has the potential to directly address the genetic abnormalities that drive cancer development. By precisely cutting and repairing DNA, CRISPR-Cas9 can disable oncogenes, those genes that promote uncontrolled cell growth, or correct tumor suppressor gene mutations, restoring their crucial function in preventing cancer. This approach promises to be far more targeted and potentially less toxic than current therapies.
The development of CRISPR-Cas9-based therapies for cancer is rapidly advancing. Researchers are exploring various strategies, including delivering the CRISPR components directly to tumor cells, utilizing viral vectors for delivery, and even engineering immune cells to carry the gene-editing machinery. Each method presents unique challenges and opportunities, and the future of this approach looks promising.
Targeting Specific Cancer Pathways
Cancer is not a single disease but rather a collection of diseases, each driven by unique genetic alterations and cellular pathways. Gene editing technologies like CRISPR allow us to target these specific pathways. By identifying the critical genes and pathways involved in a particular cancer type, researchers can develop therapies that disrupt these pathways, inhibiting tumor growth and progression. This personalized approach could significantly improve treatment efficacy and reduce side effects.
Delivery Systems for Gene Editing
Effectively delivering CRISPR-Cas9 components to target cancer cells is a critical step in making gene editing therapies a reality. Researchers are exploring various delivery methods, including viral vectors, nanoparticles, and even using the body's own immune system to carry the gene-editing machinery. Each method has its own advantages and disadvantages in terms of efficiency, safety, and specificity. Overcoming these delivery challenges is a key focus in the development of these therapies.
Off-Target Effects and Safety Concerns
Although CRISPR-Cas9 holds immense promise, potential off-target effects, where the gene-editing machinery unintentionally modifies other parts of the genome, remain a concern. Scientists are actively working to improve the precision of CRISPR-Cas9 and develop strategies to minimize off-target effects. Rigorous testing and safety protocols are crucial to ensure that these therapies are both effective and safe for patients. Long-term safety data is essential to fully assess the potential risks of CRISPR-based cancer therapies.
Beyond Single-Gene Targeting
Current gene editing strategies often focus on targeting a single gene or a few specific genes. However, cancer development is often a complex process involving multiple genetic alterations and interactions between different genes. Future research will likely explore more comprehensive approaches, targeting multiple genes or pathways simultaneously to achieve more effective and durable therapeutic outcomes. This expanded strategy could lead to more holistic and personalized treatments for various cancers.
Ethical Considerations and Future Directions
The development and application of gene-editing technologies for cancer treatment raise significant ethical considerations. Discussions about access, equity, and the potential long-term consequences of altering the human genome are crucial. The future of gene editing in oncology will depend on careful consideration of these ethical implications, while simultaneously pushing the boundaries of scientific research to develop safer and more effective cancer therapies. Continued collaboration between researchers, clinicians, and ethicists will be essential to navigate these complexities and ensure responsible and equitable access to gene editing treatments.
Current digital identity systems often suffer from fragmentation and lack of interoperability. Users are frequently required to create and manage multiple accounts across various platforms, leading to a confusing and cumbersome experience. This fragmented approach also creates significant security vulnerabilities, as individual systems may not adhere to consistent security protocols, leaving user data exposed to risks. Furthermore, these systems often lack the necessary transparency and control for users to manage their digital identities effectively.
Improving the Efficacy of Existing Therapies Through Gene Editing
Optimizing Existing Processes
A crucial aspect of enhancing efficacy lies in meticulously evaluating and optimizing existing processes. This involves a deep dive into current workflows, identifying bottlenecks, and pinpointing areas where inefficiencies are prevalent. By streamlining these processes, we can eliminate unnecessary steps and wasted resources, leading to significant improvements in overall output and productivity. This analysis often necessitates a thorough review of documentation, data, and feedback from personnel involved in the existing processes.
Detailed process mapping is a powerful tool in this optimization process. Visual representations of the steps involved allow for a clearer understanding of the flow and potential points of friction. This, in turn, enables the development of targeted solutions to improve efficiency and eliminate redundant tasks, leading to a more streamlined and effective workflow.
Leveraging Technology
Integrating appropriate technology can substantially boost the efficacy of existing systems. Modern tools and software solutions can automate repetitive tasks, providing a significant boost in efficiency and reducing the risk of human error. This can free up personnel to focus on more strategic and creative endeavors, ultimately contributing to a more productive and effective overall operation.
Furthermore, data analytics can provide valuable insights into the performance of existing processes. Analyzing data collected from various sources can highlight areas needing improvement, identify trends, and enable the development of data-driven solutions for enhanced efficacy.
Investing in Training and Development
Empowering personnel with the necessary skills and knowledge is paramount to maximizing the efficacy of any system. Investing in comprehensive training programs focused on best practices and new technologies can significantly enhance the capabilities of employees, leading to improvements in productivity and quality. This includes not only technical training but also training in soft skills like communication and collaboration, which are crucial for effective teamwork.
Streamlining Communication Channels
Clear and effective communication is essential for the smooth functioning of any organization. Improving communication channels can lead to a more streamlined workflow and fewer misunderstandings, ultimately increasing the efficacy of existing processes. This includes establishing clear communication protocols, utilizing efficient communication tools, and encouraging open dialogue among team members.
Enhancing Collaboration
Encouraging collaboration among different departments and teams is crucial for enhancing the efficacy of existing processes. This can lead to a more holistic approach, incorporating diverse perspectives and expertise to identify and implement more effective solutions. Effective collaboration fosters a synergistic environment where everyone feels empowered to contribute their unique skills and knowledge. This collaboration can be facilitated through regular team meetings, cross-functional projects, and shared platforms for information exchange.
Implementing Quality Control Measures
Implementing robust quality control measures is essential for maintaining high standards and minimizing errors. This involves establishing clear quality standards, implementing effective monitoring mechanisms, and providing opportunities for continuous improvement. By consistently evaluating the quality of outputs, we can identify areas for improvement in processes and ensure that the desired level of efficacy is consistently achieved. This can lead to higher customer satisfaction and improved reputation for the organization.
Future Directions and Challenges in Gene Editing for Oncology
Harnessing CRISPR-Cas9 for Precision Cancer Therapy
The CRISPR-Cas9 system has emerged as a revolutionary tool in gene editing, offering unprecedented precision and potential for treating various cancers. Its ability to target specific DNA sequences allows for the precise correction of mutations driving oncogenesis, potentially eliminating cancerous cells while sparing healthy ones. Further research is crucial to overcome challenges like off-target effects and delivery limitations. Scientists are actively developing more sophisticated delivery methods and strategies to improve the efficacy and safety of CRISPR-Cas9 therapies in preclinical and clinical trials.
One promising avenue is exploring the use of CRISPR-Cas9 to target specific cancer stem cells (CSCs), which are often responsible for tumor recurrence. These cells possess unique characteristics, making them potentially more vulnerable to gene editing approaches. Successfully targeting and eliminating these cells could significantly improve long-term cancer remission rates. However, understanding the intricacies of CSC biology and their specific genetic vulnerabilities is critical for successful therapeutic application.
Overcoming Challenges in Gene Editing Delivery and Off-Target Effects
Despite the remarkable progress in gene editing technology, significant hurdles remain in delivering gene editing tools effectively to cancer cells and minimizing potential off-target effects. Developing safe and efficient delivery vehicles for CRISPR-Cas9 complexes is a critical area of research. Non-viral vectors, such as lipid nanoparticles, are being investigated as promising alternatives to viral vectors, which carry inherent safety concerns. Furthermore, innovative strategies for improving the specificity of CRISPR-Cas9 are essential for ensuring targeted gene editing without unwanted alterations in the genome.
Minimizing off-target effects is paramount to ensuring the safety and efficacy of gene editing therapies. Advanced techniques for evaluating and minimizing off-target cleavage are being explored, including the use of improved guide RNA design algorithms and the development of more sophisticated screening methods. These strategies are vital for translating gene editing therapies from the laboratory to clinical practice, ensuring their safety for patients.
Developing robust methods for tracking and monitoring the precise targeting of gene editing tools in vivo is another crucial area of research. Such methods would allow researchers to assess the extent of gene editing in different tissues and identify potential issues early on. This rigorous monitoring is essential for ensuring the safety and efficacy of gene editing approaches in oncology and other therapeutic applications.
Further research into the mechanisms of off-target effects and the development of strategies to mitigate them are vital for the long-term success of gene editing therapies. Understanding the interplay between the CRISPR-Cas9 system and the cellular environment is crucial to developing more precise and effective treatments.
Optimizing the delivery of gene editing components to tumor sites is a critical aspect of achieving effective therapeutic outcomes. This includes developing targeted delivery systems that can selectively accumulate in cancerous tissues, minimizing off-target effects and maximizing the therapeutic impact.