Early Experiments and Conceptualizations
The road to modern gene editing was paved with curiosity-driven discoveries long before CRISPR-Cas9 entered the picture. During the 1970s and 1980s, researchers made groundbreaking strides in decoding the language of genes, unraveling how DNA orchestrates cellular functions through RNA and protein synthesis. These foundational studies, though primitive compared to today's standards, proved that genetic material could be deliberately modified - a revelation that sparked decades of innovation. The meticulous documentation of cellular responses to foreign genetic material established critical knowledge for future precision editing techniques.
The Discovery of Restriction Enzymes
Molecular biology underwent a seismic shift when scientists uncovered nature's precision scissors - restriction enzymes. These bacterial defense mechanisms slice DNA at predictable locations, providing researchers with their first reliable tool for genetic surgery. The ability to isolate and rearrange specific DNA fragments birthed recombinant DNA technology, creating possibilities that ranged from pharmaceutical production to agricultural improvements. This watershed moment demonstrated that genetic material could be edited with purposeful intent rather than random chance.
Recombinant DNA Technology and its Impact
The marriage of restriction enzymes with DNA ligases enabled scientists to stitch together genetic material from disparate sources. While not gene editing in the contemporary sense, this capability to engineer novel genetic combinations fundamentally altered our understanding of genetic control mechanisms. The creation of the first genetically modified organisms provided tangible proof that organisms could be enhanced through deliberate genetic intervention, setting the stage for more sophisticated editing approaches.
Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs)
The turn of the millennium witnessed the emergence of programmable molecular tools that could target specific DNA sequences with unprecedented accuracy. ZFNs and TALENs represented a quantum leap in precision, though their complex protein engineering requirements limited widespread adoption. These systems demonstrated that targeted gene editing was achievable, solving the precision problem that had hampered earlier techniques. Their development crucially informed the design of next-generation editing platforms.
The CRISPR-Cas9 Revolution
CRISPR-Cas9 emerged as a democratizing force in genetic engineering, transforming what was once an arcane specialty into an accessible technology. This bacterial immune system adaptation combines remarkable precision with surprising simplicity, enabling applications from disease eradication to crop improvement. Its versatility has spawned ethical debates as profound as its scientific implications, particularly regarding human germline modifications. The technology's rapid evolution continues to outpace regulatory frameworks, necessitating ongoing ethical examination.
AI's Role in Enhancing CRISPR-Cas9 Accuracy and Efficiency
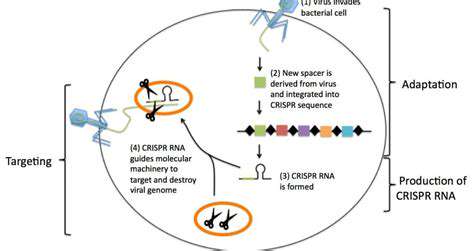
Harnessing AI for Precise CRISPR-Cas9 Editing
Artificial intelligence has become CRISPR's perfect partner, with machine learning algorithms sifting through genomic data to identify optimal editing sites. This computational power reduces off-target effects dramatically while boosting editing efficiency, making previously unthinkable precision achievable. Predictive modeling now allows researchers to preview potential outcomes before conducting physical experiments, saving months of trial-and-error work.
Predicting CRISPR-Cas9 Efficiency
The alchemy of successful gene editing depends heavily on guide RNA selection. AI transforms this from guesswork to predictable science, analyzing thousands of variables to forecast editing success rates. This predictive capability enables researchers to focus resources on the most promising candidates, accelerating therapeutic development while reducing wasted effort on ineffective constructs.
Optimizing CRISPR-Cas9 Delivery Systems
Getting CRISPR components into cells presents one of the technology's greatest challenges. AI analyzes countless delivery parameters - from nanoparticle composition to electrical pulse duration - identifying the safest, most efficient transport methods for specific cell types. This optimization is particularly crucial for in vivo applications where precision delivery can mean the difference between therapeutic success and dangerous side effects.
Identifying Off-Target Effects
AI serves as CRISPR's quality control system, scanning entire genomes to predict where unintended edits might occur. This computational safety check is revolutionizing therapeutic development, allowing researchers to redesign guide RNAs that minimize collateral genetic damage. The ability to model these risks computationally before human trials begins represents a major advance in patient safety.
Developing CRISPR-Cas9-Based Diagnostics
The marriage of CRISPR and AI is producing diagnostic tools with unprecedented speed and accuracy. Machine learning algorithms can detect disease signatures in genetic material that would elude human analysts, enabling earlier and more precise diagnoses. This synergy proves particularly valuable for complex conditions with subtle genetic markers, where early detection significantly improves outcomes.
AI-Driven Drug Discovery and Personalized Medicine
AI's Impact on Target Identification
The traditional drug discovery process - often compared to finding a needle in a haystack - is being revolutionized by AI's pattern recognition capabilities. Machine learning algorithms can identify promising drug targets in days rather than years, analyzing interactions across vast biological networks that human researchers could never process manually. This accelerated target identification is particularly valuable for rare diseases where traditional research economics have failed patients.
Personalized Medicine Approaches Enabled by AI
AI transforms the one-size-fits-all treatment paradigm by analyzing individual genetic blueprints. This computational approach predicts how specific patients will respond to therapies, allowing clinicians to customize treatment plans with unprecedented precision. The technology considers not just genetics but lifestyle factors and treatment histories, creating truly personalized care strategies that maximize efficacy while minimizing adverse effects.
Accelerated Drug Development Through Machine Learning
Pharmaceutical development timelines are collapsing as machine learning optimizes every step - from molecular design to clinical trial planning. AI models can predict compound behavior with accuracy rivaling physical experiments, enabling virtual screening of millions of candidates. This computational power also designs smarter clinical trials that require fewer participants while generating more definitive results, potentially shaving years off development cycles.