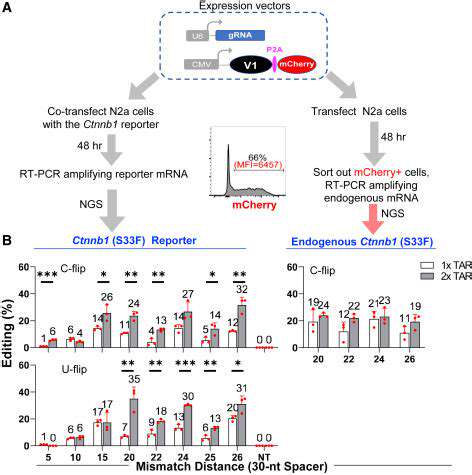
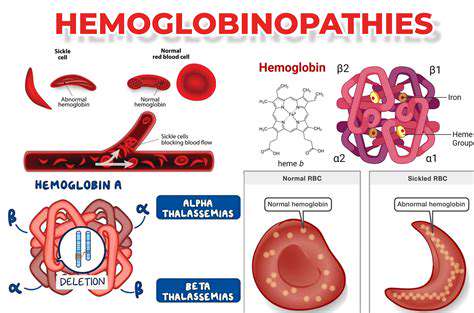
Harnessing Cellular Factories for Tailored Therapies
Harnessing the Power of Cellular Metabolism
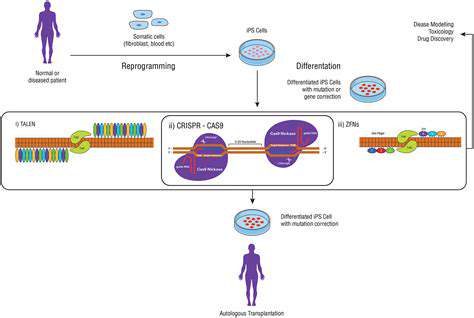
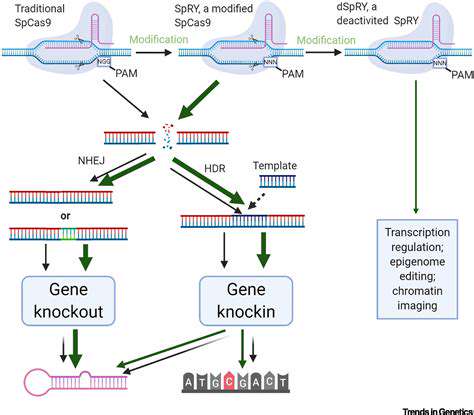
Cellular metabolism, the intricate network of biochemical reactions within cells, is a powerful engine for creating a wide array of molecules. Understanding and manipulating these metabolic pathways is crucial in synthetic biology, enabling us to engineer cells to produce specific compounds, not just for basic research but also for applications in personalized therapies. This includes developing cells that can generate drugs, produce biomaterials, or even deliver targeted therapies directly to diseased tissues.
By carefully selecting and modifying metabolic pathways, scientists can reprogram cells to prioritize the synthesis of desired molecules. This process involves introducing new genes, modifying existing ones, or even eliminating unwanted pathways. The result is a cellular factory capable of producing tailored therapies with unprecedented precision.
Designing Specific Cellular Responses
A key aspect of harnessing cellular factories for tailored therapies involves designing cells to respond to specific stimuli or signals. This allows for highly targeted delivery of therapies, minimizing side effects and maximizing efficacy. For example, cells can be programmed to release therapeutic compounds only when they detect the presence of a specific molecule associated with a disease, thereby avoiding unnecessary exposure of healthy tissues.
This targeted approach is especially crucial in cancer treatment. Imagine cells that recognize and specifically target cancer cells, releasing therapeutic agents only at the tumor site. This personalized approach reduces the risk of harm to healthy cells and improves the effectiveness of the treatment.
Engineering Cellular Delivery Systems
Beyond producing therapeutic molecules, cells can be engineered to act as sophisticated delivery systems. By incorporating specific targeting mechanisms, cells can be directed to specific tissues or organs, ensuring that the therapeutic agents reach the desired location with minimal side effects. This targeted delivery system can also include the ability to release drugs in a controlled manner over time, ensuring consistent and effective treatment.
This aspect of cellular engineering holds immense potential for treating chronic diseases. Imagine cells that can deliver anti-inflammatory drugs directly to inflamed tissues or cells that release insulin in a controlled manner for managing diabetes. This approach offers a novel and personalized approach to managing a wide range of diseases, surpassing traditional methods.
Developing Personalized Therapies
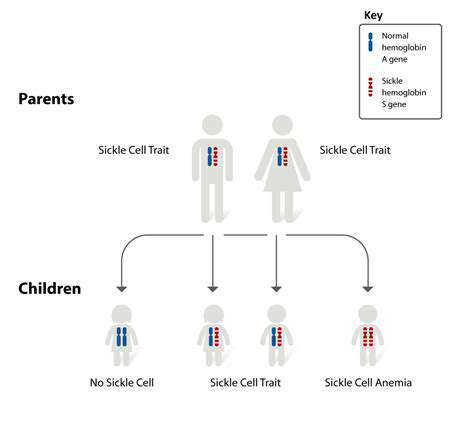
The ultimate goal of harnessing cellular factories is to develop personalized therapies tailored to the individual needs of each patient. By analyzing a patient's unique genetic makeup and disease characteristics, we can engineer cells to produce therapies that are highly specific and effective in combating their particular disease. This approach promises to revolutionize healthcare, offering treatments that are more effective, less toxic, and better suited to individual needs.
This personalized approach is not limited to drug production; it extends to the development of biomaterials for tissue regeneration, and even to the design of immune cells that specifically target cancer cells in a patient. The potential for personalized medicine is truly transformative, offering hope for a future where treatments are as unique as the patients themselves.

Customizable Bioreactors for Drug Production

Choosing the Right Bioreactor
Selecting the appropriate bioreactor for a specific drug development project is crucial for achieving optimal results. This decision hinges on factors like the desired scale of production, the type of cell culture being used, and the specific properties of the drug being manufactured. Careful consideration must be given to the bioreactor's ability to maintain a stable and controlled environment, ensuring the health and productivity of the cells.
Different bioreactor configurations, from stirred-tank to airlift, each have unique advantages and disadvantages. Understanding these nuances is essential for selecting the most suitable option for the task at hand. Factors such as mixing efficiency, oxygen transfer capabilities, and ease of cleaning and sterilization should be weighed carefully.
Material Selection and Sterilization
The materials used in the construction of a bioreactor significantly impact the safety and efficacy of the drug production process. Materials must be compatible with the cell culture and the specific drug being produced, avoiding any potential contamination or adverse reactions. Stainless steel is a common choice due to its inertness and resistance to corrosion, but other materials might be preferred depending on the specific application.
Sterilization procedures are critical to maintaining the sterility of the bioreactor system throughout the production process. Implementing rigorous sterilization protocols, such as steam sterilization or hydrogen peroxide treatments, is vital to prevent contamination and ensure the quality of the final product.
Control Systems and Monitoring
Sophisticated control systems are essential for maintaining optimal conditions within the bioreactor. These systems allow for precise monitoring and adjustment of parameters like temperature, pH, dissolved oxygen, and nutrient levels. Constant monitoring and feedback loops are required to ensure that the culture remains healthy and productive throughout the process.
Automated systems provide real-time data and allow for rapid adjustments to maintain the desired conditions, ultimately increasing production efficiency. Advanced sensors and data analysis tools are becoming increasingly important for optimizing bioreactor performance and achieving consistent drug quality.
Scale-Up Strategies
Scaling up bioreactor operations from laboratory settings to industrial-scale production is a critical aspect of drug manufacturing. Transitioning from small-scale prototypes to larger production units requires careful consideration of process parameters and equipment modifications.
Strategies for scale-up must minimize risks of contamination and ensure consistent product quality across different scales. Proper consideration of mixing, aeration, and heat transfer is essential for successful scale-up.
Process Optimization for Enhanced Productivity
Optimizing the bioreactor process is vital for maximizing drug production yields and minimizing costs. This involves analyzing various factors such as media formulations, cell densities, and operating parameters to identify areas for improvement.
Implementing process optimization techniques can lead to significant improvements in productivity and overall efficiency of drug manufacturing. Data analysis and modeling are crucial tools for identifying and addressing bottlenecks in the bioreactor process.
Cost-Effectiveness of Customization
The cost of customizing a bioreactor can vary significantly depending on the complexity of the design and the specific requirements of the project. However, the benefits of tailored bioreactors can often outweigh the initial investment.
A customized bioreactor can be more efficient and cost-effective in the long run by optimizing production and reducing waste. This is particularly true when the bioreactor is designed to meet the specific needs of a particular drug or cell line.
Regulatory Compliance and Safety
Adherence to strict regulatory guidelines and safety protocols is paramount in the development and operation of bioreactors for drug production. This includes adhering to regulations related to biomanufacturing, product quality, and safety standards.
Maintaining rigorous documentation of all processes and procedures is essential for regulatory compliance and ensures traceability of the drug production process. Safety measures must be implemented to prevent accidents and ensure the well-being of personnel working with the bioreactor.
Ethical Considerations and Future Directions
Ethical Considerations in Personalized Medicine
The application of synthetic biology to personalized medicine presents a complex web of ethical considerations. Ensuring equitable access to these potentially transformative therapies is paramount. The potential for genetic discrimination and the creation of a two-tiered healthcare system based on genetic predispositions must be carefully addressed through robust regulatory frameworks and societal dialogue. We must prioritize ethical guidelines that protect patient privacy and autonomy while maximizing the benefits of this innovative technology.
Furthermore, the potential for misuse of this technology, including the creation of designer babies or the enhancement of human capabilities beyond their natural limits, demands careful scrutiny. Open and transparent discussions about societal values and the long-term implications of these advancements are crucial to navigating the ethical landscape of personalized medicine powered by synthetic biology.
Data Security and Privacy Concerns
The collection and analysis of vast amounts of genetic data in personalized medicine create significant data security and privacy concerns. Robust security protocols and encryption methods are essential to protect patient information from unauthorized access and breaches. Strong legal frameworks and ethical guidelines are needed to regulate the storage, use, and sharing of this sensitive data, minimizing potential harm and maximizing the benefits of this technology for individuals and society.
The potential for data breaches and misuse of genetic information by individuals or institutions must be addressed through rigorous safety protocols and transparent data handling practices. This includes ensuring that patients have control over their data and understand how it is being used and protected. Transparency and patient empowerment are crucial elements in building trust and addressing these concerns.
Accessibility and Equity in Personalized Medicine
Ensuring equitable access to personalized medicine therapies developed using synthetic biology is critical. The high costs associated with these treatments could exacerbate existing health disparities, creating a divide between those who can afford these advanced therapies and those who cannot. Strategies for affordability and accessibility, such as subsidized programs or innovative funding models, are vital to ensure that the benefits of personalized medicine are available to all individuals, regardless of their socioeconomic status.
Long-Term Implications and Societal Impact
The long-term implications of synthetic biology for personalized medicine extend far beyond individual patients. These advancements may reshape healthcare systems, research methodologies, and even our understanding of human biology. Careful consideration must be given to the potential societal impact, including the potential for unforeseen consequences and the need for ongoing monitoring and adaptation of policies and practices as the field evolves.
The evolving nature of synthetic biology and its applications in personalized medicine necessitate ongoing dialogue and adaptation. This includes engaging with diverse stakeholders, including patients, researchers, policymakers, and the public, to ensure that these advancements align with societal values and contribute to a more equitable and sustainable future for all.
Regulatory Frameworks and Governance
Robust regulatory frameworks are essential to govern the development and application of synthetic biology in personalized medicine. Clear guidelines are needed to ensure that research and clinical trials adhere to ethical standards and protect patient safety. These regulations should also address the need for continuous evaluation and adaptation as the technology evolves. Establishing transparent and accountable governance structures is crucial to mitigating potential risks and maximizing the benefits of this emerging field.