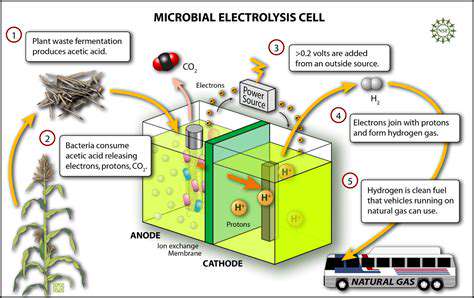
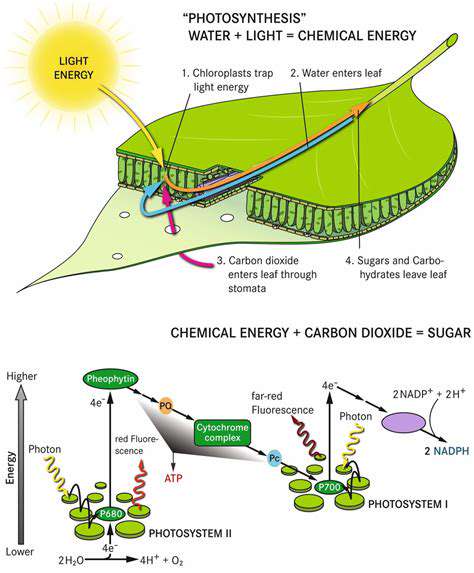
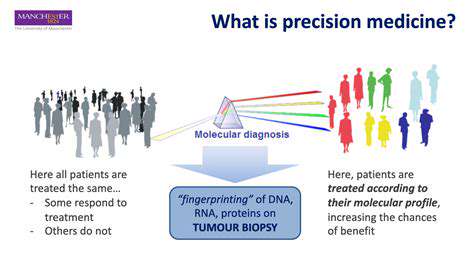
Modern enterprises are revolutionizing customer interactions through cutting-edge artificial intelligence solutions. These technologies have evolved from speculative concepts to indispensable assets, powering personalized recommendations and intelligent support frameworks. The latest generation of customer relationship platforms leverages unprecedented algorithmic complexity, enabling organizations to deliver bespoke experiences while optimizing operational outcomes. Mastery of core AI methodologies, particularly machine learning architectures and neural network paradigms, forms the foundation of this paradigm shift.
Novel Nucleases from Untapped Biological Sources: Expanding the Toolkit
Exploring the Diversity of Untapped Biological Sources
Microbial ecosystems represent a largely uncharted frontier for enzyme discovery, particularly concerning nucleases with specialized functions. These biological catalysts govern essential genetic processes across diverse lifeforms, frequently demonstrating extraordinary molecular precision. The systematic investigation of these biological treasure troves represents a pivotal endeavor for advancing biotechnological innovation and medical breakthroughs. Rigorous analysis of microbial populations - ranging from terrestrial microbiomes to extreme habitats - continues to uncover nucleases with unprecedented catalytic properties.
Extremophilic organisms, thriving in seemingly inhospitable environments, have evolved enzymes capable of maintaining activity under conditions that would denature conventional proteins. Such remarkable biochemical resilience makes these molecular machines particularly valuable for industrial processes requiring operation in non-standard environments.
Unveiling Novel Nucleotide Recognition Mechanisms
The molecular architecture of nucleases enables exquisite specificity in DNA sequence identification and cleavage. Deciphering these recognition paradigms is fundamental both for creating precision genetic engineering tools and for expanding our comprehension of cellular biology. Emerging nuclease variants may introduce novel binding configurations, potentially rewriting current models of nucleic acid interaction.
Nucleases exhibiting unconventional substrate preferences could spawn innovative diagnostic methodologies and treatment modalities. Through comprehensive characterization of their target discrimination mechanisms, we may unlock new possibilities for controlled genetic manipulation.
Optimizing Nucleases for Biotechnology Applications
Next-generation nucleases offer transformative potential for biotechnological innovation, particularly in genome engineering and molecular diagnostics. Their distinguishing attributes - including enhanced specificity profiles, thermal stability, and catalytic efficiency - promise to redefine industry standards. The capacity for high-fidelity sequence targeting represents a critical enabler for advanced gene therapy development.
Protein engineering approaches can further refine these enzymes for specialized applications. Strategic modifications to structural elements or incorporation of novel functional modules may significantly enhance performance characteristics for targeted use cases.
Examining Nucleases for Potential Medical Applications
Specialized nucleases capable of identifying disease-associated genetic markers could revolutionize clinical practice. Their application spans from early detection of pathogenic nucleic acids to corrective genomic interventions for hereditary conditions, potentially ushering in a new era of precision medicine. The development of sequence-specific DNA cleavage technologies continues to expand therapeutic possibilities.
Addressing Challenges in Nucleases' Characterization and Engineering
The systematic isolation and analysis of novel nucleases from complex biological samples presents significant technical hurdles. Implementing high-throughput screening methodologies proves essential for identifying enzymes with optimal characteristics. This necessitates both refinement of existing protocols and creation of innovative analytical frameworks to facilitate large-scale enzyme characterization.
Directed evolution of novel nucleases for enhanced functionality represents a critical research priority. Successful engineering efforts demand comprehensive understanding of structural determinants governing enzymatic activity. Development of sophisticated computational models and experimental techniques will be instrumental in accelerating progress.
Future Directions and Potential Impact
Prospecting for novel nucleases in understudied biological systems holds extraordinary promise. Sustained exploration of microbial diversity coupled with advancements in screening and protein engineering technologies will undoubtedly yield transformative discoveries. The implications of these findings extend across multiple disciplines, from medical science to agricultural biotechnology.
Potential applications transcend genome editing, encompassing environmental biotechnology and industrial manufacturing sectors. These scientific advancements are positioned to redefine technological capabilities across multiple domains.