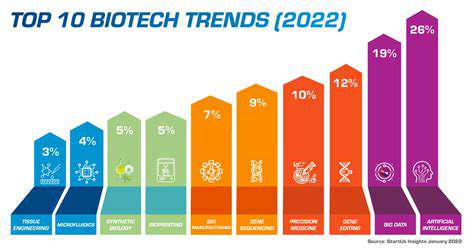
Introduction to Synthetic Biology in Enzyme Engineering

What is Synthetic Biology?
Synthetic biology is a rapidly evolving field of science that combines engineering principles with biological systems. It involves designing and constructing new biological parts, devices, and systems, or modifying existing ones for specific purposes. This interdisciplinary approach draws upon expertise from various fields, including biology, engineering, computer science, and chemistry. At its core, synthetic biology seeks to understand and harness the power of biological systems to address global challenges and create innovative solutions.
This exciting field offers enormous potential for advancing various sectors, from medicine to agriculture. Researchers can reprogram cells to produce valuable compounds, develop new diagnostic tools, and create more sustainable methods for producing energy.
Key Concepts in Synthetic Biology
Central to synthetic biology are the concepts of modularity and standardization. Researchers design biological components, often called parts, in a way that they can be combined and recombined to create complex systems. This modular approach enables scientists to build and test different designs efficiently and predictably, much like designing a circuit using standardized components.
Another crucial concept is the ability to program biological systems. This involves using genetic circuits to control the behavior of cells, similar to how computer code controls the actions of a computer. These genetic circuits can regulate gene expression, cell signaling, and metabolic pathways, providing precise control over the cellular processes.
Applications of Synthetic Biology
The applications of synthetic biology are vast and span numerous fields. In medicine, for example, synthetic biology is being used to develop new therapies for diseases such as cancer and diabetes. Researchers are also working on creating biofactories to produce drugs and other pharmaceuticals more efficiently and sustainably.
Furthermore, synthetic biology is revolutionizing agriculture by creating crops with enhanced nutritional value and increased resilience to environmental stress. Scientists are also exploring the potential of synthetic biology to produce biofuels and other renewable energy sources.
Ethical Considerations
As with any rapidly advancing field, synthetic biology raises important ethical considerations. The potential for misuse of these technologies must be carefully evaluated and addressed. The long-term impacts of modifying biological systems need careful consideration, and the development of appropriate regulations and guidelines is crucial. Open discussions and collaboration among scientists, policymakers, and the public are essential to ensure responsible innovation.
The Future of Synthetic Biology
The future of synthetic biology is bright, with the potential to revolutionize numerous fields. Continued research and development in this area are likely to lead to even more sophisticated tools and technologies, offering innovative solutions to global challenges. The development of new and improved design tools for biological systems, along with a deeper understanding of complex biological interactions, will undoubtedly drive further progress in this field.
The potential for synthetic biology to impact areas like sustainable energy, medicine, and agriculture is extraordinary. We can anticipate new discoveries and applications that were previously unimaginable.
Tools and Technologies in Synthetic Biology
Several key tools and technologies underpin the advancements in synthetic biology. These include DNA synthesis, genetic engineering techniques such as CRISPR-Cas9, and advanced microscopy for visualizing and analyzing biological systems. These tools allow scientists to design, construct, and analyze complex biological systems with unprecedented precision and efficiency.
Computational modeling and simulation play a critical role in designing and optimizing biological systems, providing a framework for predicting outcomes and guiding experimental design. These computational tools are essential for navigating the complexity of biological systems.
Optimizing Enzyme Production through Metabolic Engineering

Improving Enzyme Stability
Enzyme stability is a crucial factor in optimizing production yields. Maintaining optimal enzyme activity over extended periods is vital for maximizing the efficiency of biocatalytic processes. Factors like temperature, pH, and the presence of stabilizing agents significantly influence enzyme stability. Careful consideration of these parameters during the production process can lead to substantial improvements in overall enzyme yield.
Minimizing exposure to denaturing conditions, such as extreme temperatures or pH fluctuations, is essential. Utilizing specific buffers and additives designed to protect enzymes from these detrimental effects can dramatically increase the operational lifespan of the enzyme, leading to a significant increase in overall production.
Optimizing Fermentation Conditions
Fermentation conditions play a critical role in the successful production of enzymes. Properly controlling parameters such as temperature, aeration, and nutrient supply is essential for maximizing enzyme yield and ensuring optimal cellular growth. Precise control of these factors can greatly enhance the efficiency of the fermentation process.
The specific needs of the enzyme-producing microorganism must be considered. Different microorganisms have varying optimal growth conditions. Understanding these requirements and tailoring the fermentation process accordingly is crucial for achieving high enzyme productivity. Maintaining a suitable substrate concentration, along with precise aeration rates, can significantly affect the final enzyme product yield.
Strain Engineering for Enhanced Production
Genetic engineering techniques can significantly enhance enzyme production. By introducing genes encoding for enhanced enzyme production or modifying existing genes, researchers can manipulate the metabolic pathways of the producing organism. This approach can lead to significantly higher enzyme yields.
Targeted mutations and the introduction of heterologous genes can dramatically increase the production rate and efficiency of the enzyme of interest. This approach allows for the development of more robust and productive enzyme-producing strains.
Furthermore, screening for naturally occurring, high-producing strains can complement these genetic engineering efforts. This involves identifying and isolating strains with inherent capabilities to produce higher quantities of the desired enzyme.
Process Scale-up and Purification Strategies
Scaling up the enzyme production process from a laboratory setting to an industrial scale requires careful consideration of various factors. Maintaining consistent enzyme activity and yield during the scale-up process is critical. Different techniques and equipment must be employed to ensure cost-effectiveness and efficiency.
Efficient downstream processing strategies for enzyme purification are crucial for obtaining a high-quality product. Optimizing purification methods, such as chromatography, can significantly reduce costs and improve the overall efficiency of the entire process. This ensures that the final product meets the required quality standards and yields a cost-effective enzyme production process.
Applications of Synthetically Engineered Enzymes in Industry
Enzyme-Catalyzed Reactions in Chemical Synthesis
Synthetically engineered enzymes are revolutionizing chemical synthesis by enabling highly specific and efficient reactions. These enzymes, often derived from naturally occurring proteins, can be modified through directed evolution or rational design to enhance their catalytic activity, substrate specificity, and stability. This allows for the development of new pathways for producing valuable chemicals, pharmaceuticals, and materials with significantly reduced environmental impact compared to traditional chemical processes. The ability to precisely control enzyme function is a key advantage, leading to increased yields and minimized waste.
Biofuel Production and Conversion
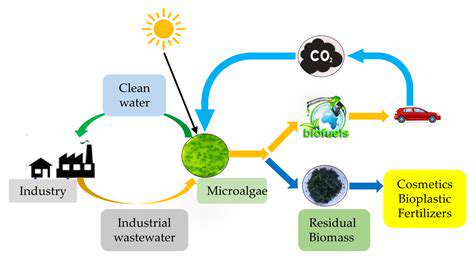
The production of biofuels, crucial for transitioning away from fossil fuels, can be significantly enhanced by utilizing engineered enzymes. These enzymes can catalyze the conversion of biomass feedstocks into biofuels like ethanol and biodiesel more efficiently and with less energy consumption. For instance, enzymes can break down cellulose, a major component of plant biomass, into fermentable sugars, increasing the overall yield of biofuels. Further advancements involve engineering enzymes to improve the conversion of complex carbohydrates into desirable biofuel molecules, reducing costs and making the process more sustainable.
Bioremediation and Environmental Cleanup
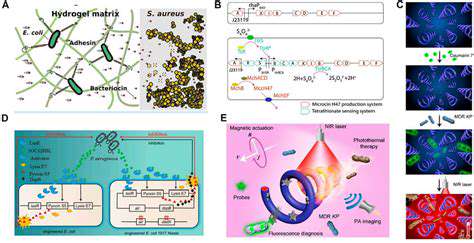
Synthetic enzymes can play a vital role in tackling environmental pollution. Engineered enzymes can be designed to degrade harmful pollutants, such as pesticides, industrial chemicals, and plastics. Their high specificity and efficiency allow for targeted degradation, minimizing the risk of creating secondary pollutants. This bioremediation approach offers a sustainable and environmentally friendly method for cleaning contaminated soil and water, reducing reliance on traditional, often harsh, remediation techniques.
Food and Beverage Industry Applications
The food and beverage industry benefits greatly from the application of synthetically engineered enzymes. These enzymes can be used for various processes, including improving the texture and flavor of food products, enhancing the digestibility of food, and producing specific ingredients for various products. For example, enzymes can be used to modify starch for improved processing, or to produce specific flavors and textures in dairy products. The precision and control offered by engineered enzymes can lead to improved efficiency and quality in food production.
Diagnostics and Therapeutics
Synthetically engineered enzymes hold immense potential in the development of diagnostics and therapeutics. Engineered enzymes can be used to develop highly sensitive and specific diagnostic tools for diseases by catalyzing reactions that produce detectable signals. Furthermore, these enzymes can be utilized as components of drug delivery systems or as therapeutic agents themselves. For example, engineered enzymes can be targeted to specific cells or tissues to deliver drugs or to degrade harmful biological components, offering a promising avenue for personalized medicine.
The Future of Enzyme Production with Synthetic Biology
Harnessing Microbial Factories for Enhanced Enzyme Production
Synthetic biology is revolutionizing the production of enzymes, moving beyond traditional methods to engineer microbial cells as highly efficient biofactories. This approach allows for the optimization of enzyme production pathways, enabling the creation of microorganisms capable of producing desired enzymes in high yields and with enhanced stability. By manipulating genetic circuits within microorganisms, scientists can fine-tune metabolic processes, leading to a significant increase in enzyme output compared to conventional methods. This targeted approach also opens doors to producing enzymes with specific properties, tailored for various industrial applications.
The ability to engineer microbial cells to produce customized enzymes holds immense potential for industries like pharmaceuticals, food processing, and bioremediation. These engineered microorganisms can be cultivated in cost-effective bioreactors, offering a sustainable alternative to traditional enzyme production methods. This efficiency extends to reducing production costs and minimizing environmental impact, making synthetic biology a game-changer in enzyme manufacturing.
Improving Enzyme Stability and Activity through Genetic Modification
A crucial aspect of enzyme production is ensuring the stability and activity of the final product. Synthetic biology allows for targeted modifications to the enzyme's amino acid sequence, leading to enhanced stability in diverse environments. This is particularly important for industrial applications where enzymes need to function effectively under varying temperatures, pH levels, and solvent conditions. By introducing specific mutations or incorporating stabilizing elements, the enzymes' robustness can be significantly improved.
Beyond stability, synthetic biology enables the enhancement of enzyme activity. Scientists can modify the enzyme's active site to improve its binding affinity to substrates, resulting in faster reaction rates and higher efficiency. These modifications, often achieved through directed evolution techniques, are crucial in optimizing enzyme performance for various industrial applications, including biofuel production and textile processing. This capability to fine-tune enzyme properties is a powerful tool for enhancing industrial processes.
Developing Novel Enzyme Catalysts for Specific Applications
Traditional enzyme production often relies on naturally occurring enzymes, limiting the range of functionalities available. Synthetic biology opens up the possibility of developing entirely new enzymes with customized catalytic properties. This approach involves designing novel enzyme structures based on computational models and then constructing them within microbial hosts. This approach is particularly useful in cases where no suitable natural enzyme exists for a specific chemical transformation. This ability to design new enzyme catalysts is paving the way for novel applications in areas like the production of fine chemicals and pharmaceuticals.
One exciting application of this capability involves creating enzymes capable of catalyzing reactions that are currently impossible or inefficient using conventional methods. This includes reactions that require specific stereochemical control or operate under extreme conditions. The ability to design enzymes from scratch holds immense promise for revolutionizing various industrial sectors, offering highly specific and efficient solutions to complex problems.
Scaling Up Enzyme Production for Industrial Applications
The success of synthetic biology in enzyme production hinges on the ability to scale up production for industrial applications. Efficient bioreactor design and optimization are crucial for achieving high enzyme yields at an economical scale. Researchers are investigating innovative bioreactor configurations and fermentation strategies to maximize enzyme production while maintaining product quality. This involves optimizing factors such as nutrient supply, temperature, and pH to ensure optimal enzyme activity and expression within the microbial hosts.
Furthermore, the development of robust downstream processing techniques is essential for isolating and purifying the enzyme from the fermentation broth. These techniques must be optimized for efficiency and cost-effectiveness to ensure the economic viability of synthetic biology-based enzyme production. This scaling up process is critical for translating laboratory discoveries into commercially viable applications.
Addressing Ethical and Regulatory Considerations
As synthetic biology advances in enzyme production, it is crucial to address the ethical and regulatory implications. Concerns regarding the potential for unintended consequences, such as the release of genetically modified organisms into the environment, must be carefully considered. Stringent safety protocols and regulations are necessary to mitigate these risks and ensure responsible development and application of this technology. Public engagement and open dialogue are essential for building trust and guiding the responsible advancement of this technology.
Furthermore, considerations regarding intellectual property rights and equitable access to these technologies are vital. Transparency and collaboration are essential for ensuring that the benefits of synthetic biology-based enzyme production are accessible to a broad range of stakeholders. Developing ethical frameworks and regulatory guidelines for this emerging field is crucial for ensuring responsible innovation.