Introduction to Synthetic Biology in Chemical Production
Defining Synthetic Biology in Chemical Production
Synthetic biology, a rapidly evolving field, is fundamentally reshaping the landscape of chemical production. It involves the design and construction of new biological parts, devices, and systems, or the redesign of existing, natural biological systems for useful purposes. In the context of chemical production, this translates to harnessing the power of living organisms – bacteria, yeast, algae, and more – to synthesize valuable chemicals. This approach offers a potentially more sustainable and efficient alternative to traditional chemical processes, which often rely on fossil fuels and generate significant waste.
This innovative approach to chemical synthesis leverages the remarkable capabilities of biological systems. These natural systems are highly efficient at catalyzing reactions and producing complex molecules, often with higher selectivity and yield than traditional chemical methods. The ability to engineer and control these biological processes holds immense potential for creating environmentally friendly and cost-effective solutions for chemical production.
Engineering Microbial Factories for Chemical Synthesis
A critical aspect of synthetic biology in chemical production is the engineering of microbial factories. This involves modifying existing microorganisms, such as bacteria or yeast, to produce specific chemicals. Researchers employ genetic engineering techniques to introduce new genes or modify existing ones, enabling the microbes to produce desired molecules. This process often involves optimizing metabolic pathways, manipulating regulatory mechanisms, and creating robust production systems within the microbial host.
A key challenge in this area is to ensure the efficiency and scalability of the engineered microbial systems. The production of chemicals at an industrial scale requires optimizing the growth conditions, minimizing byproducts, and ensuring the stability of the engineered strain. Continuous advancements in genetic engineering tools and computational modeling are crucial for overcoming these challenges and developing viable industrial applications for microbial production of chemicals.
Further challenges include the optimization of fermentation processes to maximize the yield of the desired chemical, while minimizing the production of unwanted byproducts. This requires careful consideration of factors such as nutrient supply, temperature, pH, and oxygen levels in the fermentation environment. Moreover, the development of strategies to efficiently extract and purify the desired chemical from the fermentation broth is also essential.
Sustainable and Cost-Effective Chemical Production
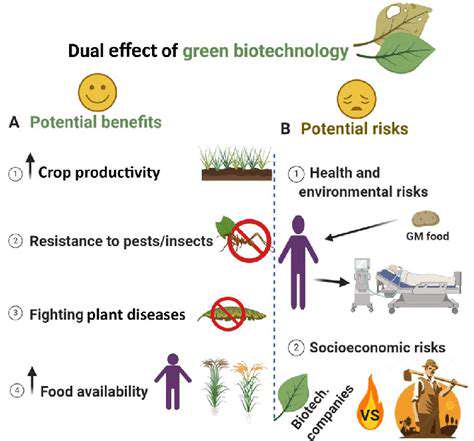
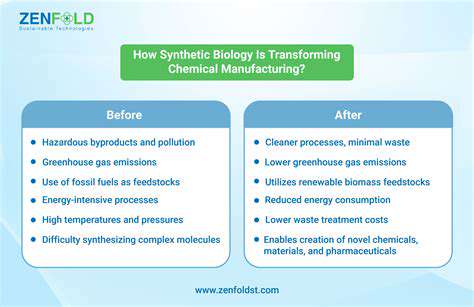
One of the most significant advantages of synthetic biology in chemical production is its potential to create sustainable processes. By utilizing renewable resources and avoiding reliance on fossil fuels, these methods have the potential to reduce the environmental footprint of chemical manufacturing. This aligns perfectly with the growing global demand for environmentally friendly and sustainable solutions to address climate change and resource depletion.
Furthermore, the inherent cost-effectiveness of synthetic biology-based approaches is a compelling factor. The use of renewable resources, coupled with the potential for high yields and reduced waste, can significantly lower the overall cost of chemical production. This is particularly important in an era where the price of fossil fuels and raw materials is constantly fluctuating.
Finally, the modularity and adaptability of synthetic biology platforms allow for the rapid development and implementation of new chemical production strategies. This enables researchers to respond quickly to evolving market demands and adjust to the needs of specific chemical applications. The dynamic nature of synthetic biology further promotes innovation and adaptability in the ever-changing chemical industry.
Harnessing Microorganisms for Chemical Synthesis
Harnessing Microbial Diversity
Microorganisms, encompassing bacteria, fungi, and archaea, exhibit an astonishing array of metabolic capabilities. Their ability to synthesize a wide range of complex molecules, from pharmaceuticals to biofuels, has long been recognized. Understanding and manipulating these natural pathways within the context of synthetic biology allows us to re-engineer these organisms for enhanced production of valuable chemicals, overcoming limitations of traditional methods and promoting sustainable industrial processes.
This diversity extends beyond just the types of microorganisms but also the vast array of metabolic pathways they possess. Each species possesses a unique genetic blueprint that dictates its functional capabilities, and this unique repertoire of enzymatic reactions can be leveraged to produce a wide range of desired compounds. Harnessing this diversity is crucial for developing robust and efficient biomanufacturing strategies.
Metabolic Engineering for Enhanced Production
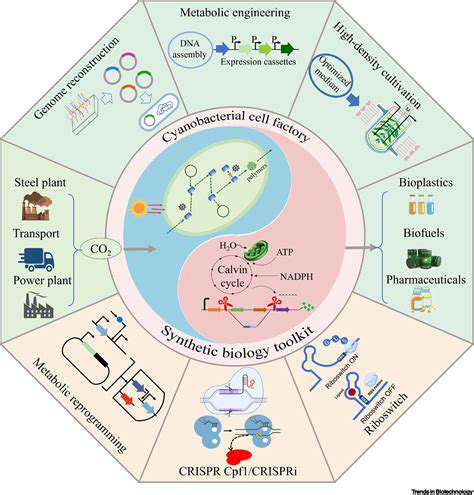
Metabolic engineering plays a critical role in optimizing microbial production of target chemicals. This involves strategically modifying the existing metabolic pathways of microorganisms to favor the production of the desired product over other cellular functions. This can include deleting pathways that compete with the target product, enhancing the expression of relevant enzymes, or introducing entirely new pathways through genetic engineering techniques.
Techniques such as gene knockout, overexpression, and pathway shuffling allow scientists to fine-tune the metabolic landscape of the microorganism, thereby significantly increasing the yield and efficiency of the desired chemical synthesis. This precision approach enables us to achieve levels of production that are difficult or impossible to attain using traditional chemical methods.
Bioreactor Design and Optimization
The efficiency of microbial chemical synthesis is not solely dependent on the genetic makeup of the organism; the environment in which it operates also plays a crucial role. Advanced bioreactor designs are essential for maximizing the productivity of microbial cultures. Optimizing factors such as temperature, pH, nutrient availability, and aeration can greatly influence the rate of product synthesis and the overall yield.
Furthermore, the scale-up of bioprocesses from laboratory settings to industrial production requires careful consideration of factors like mixing, heat transfer, and mass transfer. Developing robust and cost-effective bioreactor systems is a significant challenge, yet crucial for the practical application of synthetic biology in industrial settings.
Synthetic Biology Tools for Pathway Design
Synthetic biology provides a powerful toolkit for designing and assembling novel metabolic pathways. This includes the ability to engineer new pathways from scratch or to integrate existing microbial pathways in novel combinations. This approach allows for the production of chemicals that are not naturally synthesized by the organisms, providing access to a wider range of chemical diversity.
Computational tools play a crucial role in designing and optimizing these synthetic pathways. These tools can predict the behavior of complex metabolic networks, enabling scientists to make informed decisions about pathway design and to identify potential bottlenecks. The interplay between computational modeling and experimental validation is critical in achieving efficient and scalable biomanufacturing.
Sustainability and Environmental Impact
The use of microorganisms for chemical synthesis presents significant advantages in terms of sustainability compared to traditional chemical processes. Microorganisms can utilize renewable feedstocks, often derived from agricultural waste or other biomass, reducing reliance on finite fossil resources. Moreover, bio-based processes often produce less waste and have a significantly smaller carbon footprint.
Minimizing the environmental impact of industrial processes is paramount. By carefully selecting microbial strains and optimizing bioreactor conditions, we can strive to develop environmentally friendly and economically viable chemical synthesis pathways. This approach is essential for the responsible and sustainable growth of the synthetic biology industry.
Biocatalytic Pathways and Enzyme Engineering
Biocatalytic Pathways for Sustainable Chemical Synthesis
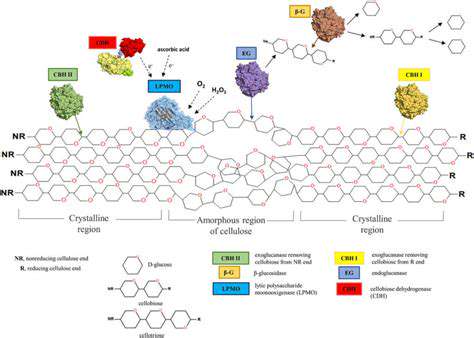
Biocatalytic pathways offer a promising avenue for sustainable chemical synthesis, leveraging the remarkable specificity and efficiency of enzymes. These pathways, often mimicking natural metabolic processes, can be engineered to produce valuable chemicals with reduced environmental impact compared to traditional chemical methods. This approach focuses on harnessing the inherent selectivity and regiospecificity of enzymes, minimizing the need for harsh reagents and byproducts frequently associated with petrochemical-based processes. By understanding and manipulating the intricacies of these pathways, we can achieve greater control over reaction outcomes and potentially lower the overall energy requirements for chemical production.
Enzyme Engineering for Enhanced Performance
Enzyme engineering plays a crucial role in optimizing biocatalytic pathways for industrial applications. This involves modifying existing enzymes or designing entirely new ones with improved properties, such as increased stability, activity, and selectivity. Techniques like site-directed mutagenesis, directed evolution, and rational design are employed to fine-tune the catalytic machinery of enzymes, allowing for tailored performance in specific chemical transformations. The goal is to engineer enzymes capable of efficiently catalyzing desired reactions at optimal temperatures and pressures, thereby enhancing the overall yield and reducing the cost of biocatalytic processes.
Metabolic Engineering for Pathway Optimization
Metabolic engineering expands on the concept of biocatalytic pathways by manipulating entire metabolic networks within an organism. This involves systematically altering the expression levels of key enzymes, or even introducing entirely new pathways, to redirect metabolic fluxes towards the production of desired compounds. By carefully controlling the flow of metabolites within the cell, we can optimize the production of target chemicals and improve the overall efficiency of the biocatalytic process. This approach often involves a deep understanding of the organism's central metabolism and the identification of critical regulatory points to fine-tune the pathway.
Designing Novel Biocatalytic Systems
The design of novel biocatalytic systems, often involving the combination of different enzymes or the incorporation of non-natural substrates, offers significant potential for expanding the scope of biocatalytic reactions. By combining enzymes from different organisms or even designing chimeric enzymes, we can access previously inaccessible reaction pathways and overcome limitations imposed by individual enzymes. This can lead to the development of entirely new biocatalytic cascades capable of producing complex molecules that are difficult or impossible to synthesize using traditional chemical methods. These novel systems can be optimized for specific conditions and substrates, ultimately leading to higher yields and better control over the overall process.
Integration of Biocatalysis into Industrial Processes
Integrating biocatalytic pathways into existing industrial processes presents a significant challenge, but also a promising opportunity for widespread adoption. This requires careful consideration of factors such as enzyme stability, scalability, and cost-effectiveness. Developing robust and efficient bioreactor designs, along with strategies for enzyme immobilization, is critical for achieving industrial-scale production. The development of efficient downstream processing techniques for isolating and purifying the desired products is also essential for making biocatalytic processes economically viable. Ultimately, the successful integration of biocatalysis into industrial settings hinges on overcoming these hurdles and demonstrating the practical advantages of this sustainable approach to chemical synthesis.
Future Directions and Challenges in Synthetic Biology for Industrial Chemical Synthesis

Emerging Technologies in the Field
The integration of artificial intelligence (AI) and machine learning (ML) is poised to revolutionize various aspects of the field, offering unprecedented opportunities for automation and data-driven insights. These technologies promise to significantly enhance efficiency and productivity, leading to more accurate and timely results. AI-powered tools can analyze vast datasets, identify patterns, and predict future trends, enabling proactive decision-making and strategic planning. This advancement will also allow for a deeper understanding of complex systems and processes.
Furthermore, the development of advanced imaging techniques, like high-resolution microscopy and 3D modeling, will provide detailed insights into cellular and molecular processes. This enhanced visualization capability is crucial for understanding disease mechanisms and developing targeted therapies. The ability to observe biological processes in unprecedented detail will undoubtedly lead to breakthroughs in medical and biological research.
Ethical Considerations and Societal Impact
As the field progresses, it's crucial to address the ethical implications and societal impacts of these advancements. The potential for misuse or unintended consequences requires careful consideration and proactive measures to ensure responsible implementation. Careful consideration of the ethical dilemmas surrounding data privacy, bias in algorithms, and equitable access to technologies is paramount. Transparency and accountability in research practices will be vital to building public trust and fostering responsible innovation.
Potential societal impacts, both positive and negative, need to be carefully evaluated and mitigated. These considerations include potential job displacement due to automation, the need for reskilling and upskilling programs, and the equitable distribution of benefits. Open dialogue and collaboration between researchers, policymakers, and the public are essential for navigating these complex issues.
Data Management and Accessibility
The exponential growth of data generated in this field demands robust data management systems and improved data accessibility. Effective strategies for data storage, organization, and retrieval are crucial for facilitating collaboration, reproducibility, and knowledge sharing. Developing standardized protocols and interoperable platforms will foster greater collaboration among researchers. This will ultimately lead to the acceleration of scientific discovery and innovation.
Resource Allocation and Funding
Securing sufficient funding and resources is essential for supporting ongoing research and development in this field. Prioritizing investment in cutting-edge technologies, skilled personnel, and infrastructure will be critical for driving progress. Attracting and retaining top talent through competitive salaries and research opportunities will ensure the field's continued success and competitiveness. The development of sustainable funding models, such as partnerships between academia, industry, and government agencies, is essential for long-term growth.
Interdisciplinary Collaboration
Future progress hinges on fostering interdisciplinary collaboration between experts in various fields. Integrating knowledge and expertise from biology, chemistry, physics, and engineering will unlock innovative solutions to complex problems. The merging of diverse perspectives and approaches can lead to groundbreaking discoveries and transformative applications. Facilitating interactions and knowledge exchange between different disciplines will be crucial for addressing the multifaceted challenges in this field.
Training and Workforce Development
Developing a skilled workforce capable of tackling the challenges of the future is vital. Investing in education and training programs that equip individuals with the necessary knowledge and skills in emerging technologies is crucial. Providing comprehensive training for students and professionals in the latest techniques and tools will allow them to contribute effectively to the field. Creating opportunities for continuous learning and professional development will be essential to maintain a competitive edge and keep pace with rapid advancements in this area.