Gene Editing for Rare Metabolic Disorders
Gene editing technologies, particularly CRISPR-Cas9, are rapidly transforming the landscape of medicine, offering unprecedented potential for treating a wide range of genetic disorders, including rare metabolic diseases. These conditions often result from mutations in specific genes, leading to a disruption in Metabolic pathways and causing debilitating symptoms. Traditional treatments for these disorders are often limited and may only provide symptomatic relief, not addressing the root cause of the disease. Gene editing holds the promise of correcting these genetic defects, potentially offering a cure or significantly improving the lives of affected individuals.
The CRISPR-Cas9 System: A Powerful Tool
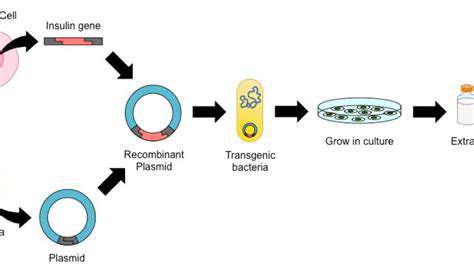
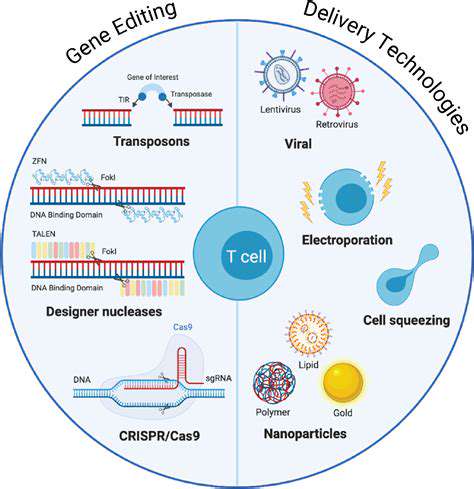
CRISPR-Cas9 is a revolutionary gene-editing tool derived from a bacterial defense mechanism. Its remarkable precision and efficiency in targeting specific DNA sequences within a genome have made it a powerful tool for researchers and clinicians. The system consists of a guide RNA molecule that directs Cas9 enzyme to the precise location of the genetic mutation. Once there, Cas9 can cut the DNA, allowing for the insertion, deletion, or modification of the genetic material. This precise targeting capability is crucial for treating rare metabolic disorders, where the goal is to repair or replace the faulty gene without causing unintended consequences.
Challenges and Future Directions
While gene editing holds immense promise, significant challenges remain. One major concern is off-target effects, where the gene-editing tool might inadvertently modify other parts of the genome. Another significant hurdle is the precise delivery of the gene-editing components to the cells or tissues affected by the metabolic disorder. Furthermore, ethical considerations surrounding gene editing need careful consideration, particularly in germline editing, where changes are passed on to future generations. Despite these challenges, ongoing research is focused on developing more precise and efficient gene-editing tools, improving delivery methods, and addressing ethical concerns, paving the way for safe and effective clinical applications in the future.
Ethical Considerations and Clinical Applications
The ethical implications of gene editing are complex and multifaceted. The potential for unintended consequences, both immediate and long-term, requires careful consideration. Public discussions and regulatory frameworks are crucial to ensure responsible development and implementation of these technologies. The application of gene editing to rare metabolic disorders is still in its early stages, but preliminary studies have shown promise in treating these conditions in animal models. Further research and clinical trials are essential to evaluate the safety and efficacy of gene editing approaches in human patients. The potential to treat diseases that have limited or no current treatment options makes it a high priority area for research and development.
Targeting Metabolic Pathways with Gene Editing
Targeting Metabolic Pathways: A Multifaceted Approach
Metabolic pathways are intricate networks of biochemical reactions that govern cellular function, energy production, and biosynthesis. Understanding these pathways is crucial for developing effective therapeutic strategies, particularly in diseases where metabolic dysregulation plays a significant role. Interfering with specific steps within these pathways can lead to the modulation of cellular processes and, potentially, the treatment of disease.
This intricate interplay of enzymes, substrates, and products makes targeting metabolic pathways a complex but potentially rewarding endeavor. Approaches range from inhibiting key enzymes to modulating the expression of genes involved in pathway regulation.
Inhibiting Key Enzymes
A fundamental strategy for targeting metabolic pathways involves the inhibition of key enzymes. These enzymes often catalyze rate-limiting steps, meaning their activity significantly impacts the overall flux through the pathway. Identifying and developing inhibitors that specifically target these enzymes is a major focus of drug discovery efforts. Careful consideration must be given to the potential for off-target effects and the development of resistance mechanisms.
The design of potent and selective inhibitors requires a deep understanding of enzyme structure and function. Computational modeling and high-throughput screening techniques are frequently used to identify promising candidates.
Modulating Gene Expression
Another approach to manipulating metabolic pathways involves modulating the expression of genes involved in pathway regulation. This approach can be used to either increase or decrease the production of specific enzymes or other proteins that control the metabolic processes. Targeting specific transcription factors or epigenetic mechanisms can alter the expression of relevant genes. This strategy offers the potential for more sustained and comprehensive metabolic modulation.
Advances in gene editing technologies, such as CRISPR-Cas9, have opened up new possibilities for precisely manipulating gene expression in cells and potentially in whole organisms, offering exciting possibilities for future therapies.
Utilizing Metabolic Intermediates as Targets
Metabolic intermediates, the molecules that are involved in the various steps of a metabolic pathway, can also be targeted for therapeutic interventions. These molecules often play crucial roles in maintaining metabolic homeostasis and their manipulation can have profound effects on cellular processes. Specific inhibitors or activators of metabolic intermediates can be developed to either block or enhance pathway activity, respectively.
The careful selection of metabolic intermediates as targets necessitates a thorough understanding of their specific functions and interactions within the broader metabolic network.
Developing Personalized Therapies
The complexity of metabolic pathways and their individual variations across different individuals necessitates a personalized approach to therapy. Metabolic profiling and genetic analysis can provide valuable insights into the specific metabolic characteristics of individual patients, enabling the development of tailored interventions. This personalized approach is crucial for maximizing therapeutic efficacy and minimizing adverse effects.
Tailored interventions are particularly important in diseases where metabolic dysregulation is a key feature. Developing personalized therapies can significantly improve treatment outcomes and reduce the risk of unwanted side effects.
Targeting Metabolic Pathways in Disease
Metabolic dysfunction is a hallmark of many diseases, including diabetes, cancer, and neurodegenerative disorders. Targeting metabolic pathways in these diseases can offer novel therapeutic strategies. By modulating specific metabolic pathways, it's possible to restore homeostasis, reduce disease progression, and improve patient outcomes.
Specific metabolic pathways implicated in these diseases can be targeted with specific inhibitors or activators, or by modulating the expression of key enzymes.
Challenges and Future Directions
Challenges in Gene Editing for Rare Metabolic Disorders
One of the significant hurdles in applying gene editing to treat rare metabolic disorders is the inherent complexity of these diseases. Many rare metabolic disorders stem from intricate genetic pathways, often involving multiple genes and proteins that interact in complex ways. Understanding these intricate networks is crucial for developing effective gene editing strategies, and current research is still actively working to fully map out and comprehend the underlying mechanisms. This complexity translates into significant challenges in designing precise and efficient gene editing tools that target the specific causative mutations without inadvertently affecting other vital genes or cellular processes.
Furthermore, achieving efficient and safe delivery of gene editing tools to the target cells within the affected tissues poses a substantial challenge. Successfully delivering these tools to the correct cells in the body, particularly in hard-to-reach tissues, remains a significant hurdle that needs to be overcome. Different delivery methods, such as viral vectors, nanoparticles, or other emerging technologies, are being explored, but optimizing their efficiency and safety for specific target tissues is an ongoing area of research.
Future Directions in Gene Editing for Rare Metabolic Disorders
Future research in gene editing for rare metabolic disorders is likely to focus on developing more precise and targeted gene editing tools, such as CRISPR-Cas9 variants with enhanced specificity and reduced off-target effects. The development of novel delivery systems that can efficiently and safely deliver gene editing tools to the specific cells and tissues affected by the disorder is another key area of advancement. Furthermore, improved understanding of the complex genetic pathways and cellular processes involved in rare metabolic disorders will likely lead to the development of more sophisticated gene editing strategies.
Additionally, the development of robust preclinical models and clinical trial protocols is essential for evaluating the safety and efficacy of gene editing therapies. These protocols need to incorporate rigorous monitoring and analysis procedures to ensure the long-term safety and efficacy of these novel therapies. This will involve extensive research and development to address the ethical considerations and potential risks associated with gene editing, particularly in the context of germline editing.
Ethical and Societal Considerations in Gene Editing
The application of gene editing technologies to treat rare metabolic disorders raises critical ethical and societal considerations. One of the most significant concerns is the potential for unintended consequences and long-term effects of gene editing interventions. Rigorous preclinical studies and clinical trials are necessary to assess the safety and efficacy of gene editing therapies, and careful monitoring of patients is crucial in the long term. The potential for gene editing to be used for non-therapeutic purposes or to create genetic inequalities must also be carefully addressed and regulated. Open discussions and collaboration among scientists, ethicists, policymakers, and the public are vital for establishing responsible guidelines and regulations for the application of gene editing technologies. This includes considering the implications for future generations and ensuring equitable access to these potentially transformative therapies.
Clinical Trials and the Road Ahead

Ethical Considerations in Clinical Trials
Clinical trials, while crucial for advancing medical knowledge and improving patient outcomes, must be conducted ethically. This involves obtaining informed consent from participants, ensuring their safety and well-being throughout the trial, and maintaining the confidentiality of their data. Protecting vulnerable populations is paramount, requiring extra scrutiny and safeguards to ensure their rights are not compromised. Researchers must adhere to strict guidelines and regulations to uphold the integrity of the trial and maintain public trust.
Furthermore, the potential for bias in trial design and data analysis must be meticulously addressed. This includes careful consideration of participant selection, blinding procedures, and data interpretation. Transparency in reporting results, both positive and negative, is essential for the advancement of medical knowledge and preventing the dissemination of misleading information.
The Role of Technology in Enhancing Clinical Trials
Technological advancements are revolutionizing the conduct of clinical trials. Remote monitoring of patients, electronic data capture, and artificial intelligence-powered analysis are streamlining processes, reducing costs, and accelerating the time it takes to gather valuable data. These innovations allow for wider recruitment of participants and more efficient data management.
Real-time data analysis can identify emerging trends and potential safety concerns, enabling quicker interventions and adjustments to the trial protocol. This can lead to more effective treatments and safer interventions for patients. Moreover, personalized medicine approaches are being incorporated into clinical trials, tailoring treatments to individual patient characteristics to enhance efficacy and minimize adverse effects.
Recruitment Strategies and Patient Engagement
Recruiting a diverse and representative group of participants is crucial for the generalizability of clinical trial results. Effective recruitment strategies that reach diverse communities are essential for ensuring inclusive participation in trials. This requires tailored communication and outreach efforts to address potential barriers to participation and build trust.
Engaging patients throughout the trial process is vital for improving compliance and retention rates. Providing clear and consistent communication about the trial's progress and any changes in the treatment protocol is paramount. Active patient feedback can provide valuable insights, shaping the trial's design and improving the overall patient experience.
Challenges and Future Directions
Despite the progress in clinical trials, significant challenges remain. Maintaining patient engagement and retention, particularly in long-term studies, can be a significant hurdle. The increasing complexity of clinical research designs, combined with the ethical considerations surrounding their implementation, requires rigorous oversight and careful planning. Ensuring equitable access to clinical trials for all populations is another key challenge that requires innovative solutions.
Future directions in clinical trials focus on developing more efficient and effective trial designs that can adapt to evolving medical knowledge. Personalized medicine approaches and the use of advanced technologies will play a crucial role in this evolution. Ultimately, the goal is to create more responsive and patient-centered clinical trials that can deliver impactful results and translate research findings into improved healthcare practices.
Funding and Resources for Clinical Trials
Adequate funding is critical for supporting high-quality clinical trials. Securing funding from various sources, including government agencies, philanthropic organizations, and industry partners, is essential for driving innovation and progress in medical research. The availability of sufficient resources for research staff, equipment, and infrastructure is vital for the successful execution of clinical trials.
The need for collaborative efforts among researchers, institutions, and funding bodies is also paramount. Strategic partnerships can leverage resources and expertise to accelerate the pace of clinical research and improve the efficiency of trial implementation. This collaborative environment fosters innovation and accelerates the translation of research findings into tangible benefits for patients.