Ethical Considerations in Patient Safety
Prioritizing patient safety goes beyond mere error avoidance—it's a profound ethical duty rooted in respecting human dignity and autonomy. This commitment must encompass emotional and psychological well-being, ensuring patients feel valued and empowered throughout their healthcare journey. Healthcare providers must honor a patient's right to refuse treatment, even when it contradicts medical advice, as this respects their personal values and self-determination.
Key ethical considerations include rigorous risk-benefit analyses for all medical interventions. Transparent communication builds trust and ensures truly informed consent. Institutions should cultivate a blame-free safety culture where errors become learning opportunities, supported by systemic improvements rather than individual reprimands. Continuous education and robust protocols are essential to mitigate the inevitability of human error.
Respecting Patient Autonomy and Dignity
At the heart of quality care lies the recognition of patient autonomy. Individuals have the fundamental right to shape their health decisions, even when diverging from clinical recommendations. Active listening to patient concerns, cultural beliefs, and personal values is non-negotiable—these factors critically influence health outcomes and satisfaction.
Effective communication forms the backbone of autonomy. Providers must deliver complex medical information in digestible formats, thoroughly explaining treatment options, risks, and alternatives. Dignity in care manifests through consistent compassion, empathy, and recognition of each patient's intrinsic worth—qualities that transform clinical encounters into healing partnerships.
Promoting a Culture of Safety and Transparency
Healthcare organizations must institutionalize safety through fail-safe systems and open communication channels. Transparent adverse event reporting enables pattern recognition and preventive strategies, while psychological safety empowers staff to voice concerns without retaliation. Such environments reduce preventable harm while fostering continuous quality improvement.
Addressing Ethical Dilemmas in Patient Care
Complex scenarios like end-of-life decisions or resource allocation demand ethical frameworks. Multidisciplinary ethics committees provide crucial guidance when values conflict, ensuring decisions align with both moral principles and individual circumstances. These deliberations must balance clinical evidence with deep respect for personal narratives.

Data Integrity and Transparency: Maintaining Trustworthiness
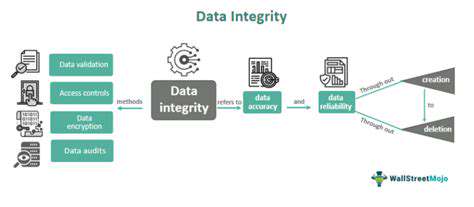
Data Integrity: A Cornerstone of Trust
Data integrity forms the foundation of organizational credibility, ensuring information remains accurate and reliable across its lifecycle. Decision-makers across industries depend on pristine data—from clinical trial results to financial forecasts—making validation protocols indispensable. Effective data governance combines technological safeguards with human accountability, creating systemic checks against corruption or misuse.
Transparency: Fostering Confidence and Collaboration
Open data practices demystify analytical processes for stakeholders. Detailed documentation of methodologies and limitations builds confidence in findings, particularly when sharing with external partners. Transparency transforms raw data into actionable knowledge while establishing organizational accountability.
Maintaining Data Integrity Through Robust Systems
Continuous data stewardship requires layered protections: automated validation, access controls, and regular audits. Comprehensive documentation ensures traceability from collection through analysis. These measures create self-correcting systems that preserve data reliability despite evolving operational demands.
The Role of Technology in Ensuring Transparency and Integrity
Modern tools like AI-driven validation and interactive dashboards enhance both data quality and accessibility. Visualization technologies bridge the gap between technical analysts and decision-makers, democratizing data-driven insights. When combined with rigorous governance, these innovations create trustworthy information ecosystems.
Regulatory Oversight and Ethical Review Boards: Maintaining Standards
Ensuring Transparency and Accountability
Independent oversight bodies serve as ethical compasses, balancing innovation with public protection. Their multi-stakeholder review processes and public commentary periods create checks against institutional bias. This rigorous scrutiny transforms abstract principles into operational safeguards.
Protecting Vulnerable Populations
Special protections for marginalized groups prevent exploitation under the guise of progress. Review boards must critically assess power dynamics and implement safeguards like independent advocates. True ethical progress is measured by how we protect those most at risk.
Promoting Informed Consent
Consent processes must evolve beyond legal checklists into meaningful dialogues. Review boards should evaluate comprehension testing methods and cultural appropriateness of materials. Genuine consent requires understanding, not just signatures.
Maintaining Data Integrity and Confidentiality
In our data-driven era, review boards must scrutinize cybersecurity measures and anonymization techniques. Breach prevention protocols are equally as important as the research itself when handling sensitive information.
Balancing Risk and Benefit
Ethical calculus requires weighing potential harms against societal gains. Boards should mandate alternative analysis when risks disproportionately affect specific populations. The most ethical solutions often emerge from this tension between progress and protection.