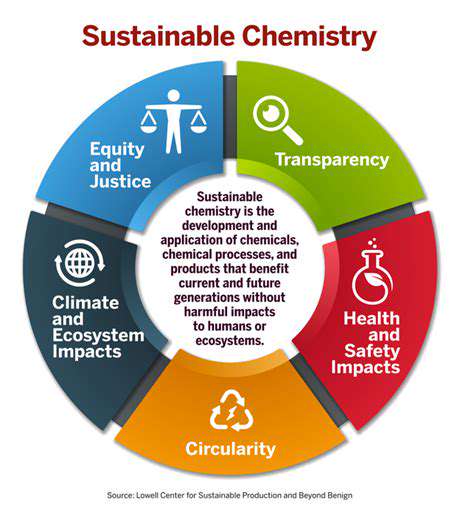
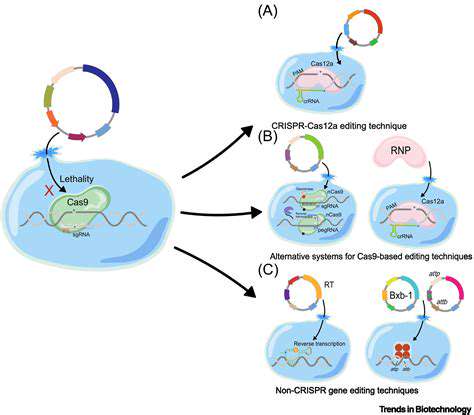
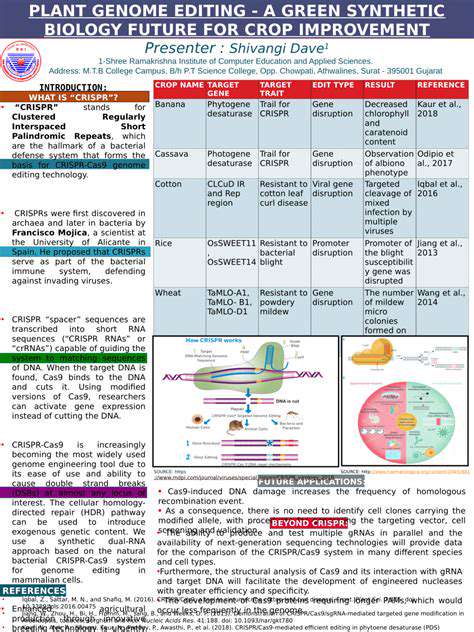
Introduction to Synthetic Biology and Renewable Chemicals
Introduction to Synthetic Biology
Synthetic biology is a rapidly evolving field that combines engineering principles with biology to design and construct novel biological systems. It involves modifying existing biological systems or creating entirely new ones to perform specific tasks, ranging from producing biofuels and pharmaceuticals to environmental remediation. This interdisciplinary approach leverages tools from molecular biology, genetics, and computer science to achieve unprecedented control over biological processes.
At its core, synthetic biology aims to understand and manipulate biological systems at a fundamental level. This knowledge allows scientists to reprogram cells and organisms to produce desired products or perform specific functions more efficiently than natural processes. This precision and control are crucial for addressing global challenges and developing innovative solutions.
The Role of Synthetic Biology in Renewable Chemicals
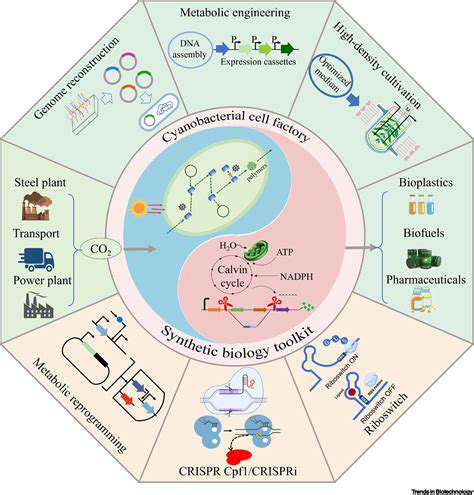
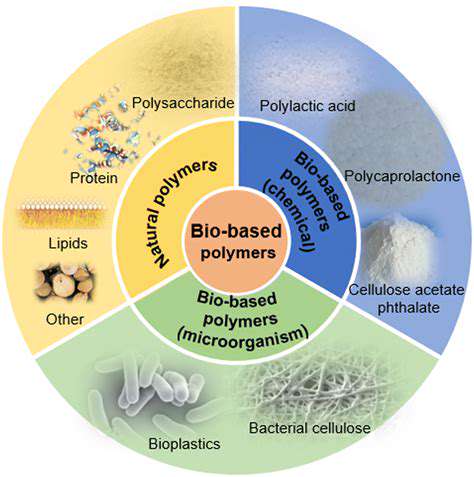
Renewable chemicals, derived from biomass rather than fossil fuels, are vital for a sustainable future. Synthetic biology plays a key role in accelerating the production of these chemicals by enabling the design and engineering of microorganisms to efficiently convert biomass into desired chemical compounds. This approach offers a significant advantage over traditional methods, as it can potentially reduce costs and environmental impact.
By incorporating synthetic pathways into microorganisms, researchers can optimize metabolic processes to maximize the yield and purity of renewable chemicals. This tailored approach is crucial for creating economically viable and environmentally friendly processes for producing chemicals like bioplastics, biofuels, and fine chemicals.
Designing Microbial Factories for Chemical Production
A critical aspect of synthetic biology in this context is the design of microbial factories. These are genetically engineered microorganisms, often bacteria or yeast, capable of efficiently converting feedstocks into the desired renewable chemicals. Sophisticated genetic engineering techniques allow scientists to introduce and modify metabolic pathways within these organisms, enabling them to produce specific chemicals at a high rate and with minimal waste.
The design process often involves computational modeling to predict the behavior of the engineered pathways and optimize their performance. This predictive modeling allows scientists to identify potential bottlenecks and refine the design to achieve the desired outcome.
Bioconversion of Biomass into Chemicals
Biomass, such as agricultural residues, algae, and wood, represents a vast and sustainable feedstock for producing renewable chemicals. Synthetic biology enables the bioconversion of these materials into valuable chemicals by engineering microorganisms to effectively break down and utilize the complex components of biomass. This process avoids the need for harsh chemical treatments, which are often environmentally detrimental.
Metabolic Engineering for Enhanced Chemical Production
Metabolic engineering is a cornerstone of synthetic biology, focusing on manipulating the metabolic pathways within organisms to enhance the production of specific chemicals. By introducing or modifying genes responsible for specific enzymatic reactions, researchers can fine-tune the metabolic flux towards the desired product, optimizing the overall yield. This process often involves the integration of multiple genes and regulatory elements to achieve a desired level of control.
Challenges and Future Directions
Despite the significant progress, challenges remain in the field of synthetic biology for renewable chemicals. One major hurdle is the scalability of these bioprocesses. Moving from laboratory-scale production to industrial-scale production presents technical and economic challenges. Further research is needed to optimize the efficiency and cost-effectiveness of these processes.
The future of synthetic biology in renewable chemicals looks promising, with ongoing developments in areas such as genetic engineering techniques, metabolic pathway optimization, and process engineering. These advancements will pave the way for the widespread adoption of bio-based chemicals, contributing to a more sustainable and environmentally friendly future.
Economic and Societal Impacts
The development of renewable chemicals through synthetic biology holds significant economic and societal implications. It has the potential to create new industries and jobs, reduce dependence on fossil fuels, and mitigate environmental impacts associated with traditional chemical production. The integration of synthetic biology into existing industrial processes will be crucial for realizing its full potential and creating a sustainable chemical sector.
Furthermore, the ethical considerations surrounding the use of synthetic biology and the potential impact on biodiversity and ecosystems need careful attention as the field progresses. Responsible development and implementation of these technologies are crucial for ensuring their positive contribution to society.
Engineering Microorganisms for Chemical Production
Harnessing Metabolic Pathways for Biomanufacturing
Engineering microorganisms to produce valuable chemicals requires a deep understanding of their metabolic pathways. Researchers can reprogram these pathways to redirect the flow of carbon and energy, enabling them to synthesize desired products. This involves identifying key enzymes, understanding their regulation, and then introducing or modifying these components to achieve optimal product yield. A critical aspect of this process is optimizing the conditions for microbial growth and product formation, including factors such as temperature, pH, and nutrient availability.
Metabolic engineering strategies are powerful tools for achieving high-throughput and cost-effective production of chemicals. By carefully manipulating the metabolic pathways of microorganisms, we can precisely control the synthesis of target compounds, leading to improved efficiency and reduced environmental impact compared to traditional chemical synthesis.
Developing Robust and Efficient Microbial Hosts
Choosing the appropriate microbial host is paramount in biomanufacturing. Factors like growth rate, substrate utilization, and product secretion capabilities significantly impact the overall process efficiency. Researchers are employing genetic engineering techniques to develop robust and efficient microbial hosts that can withstand various stresses and produce chemicals with high yields.
This includes optimizing the genetic stability of the engineered strains, minimizing the risk of genetic drift, and ensuring high levels of product secretion or accumulation. Strain optimization is a crucial step towards achieving sustainable and scalable biomanufacturing processes.
Improving Product Secretion and Recovery
Efficient secretion of the desired chemical is often a bottleneck in biomanufacturing. The produced chemical needs to be effectively exported from the microbial cell into the surrounding medium. This requires careful design of secretion signals and pathways, minimizing the intracellular accumulation of the product to prevent toxicity and improve the overall yield.
Utilizing Synthetic Biology Tools for Strain Design
Synthetic biology offers a toolbox of powerful tools for designing and constructing novel microbial strains. These tools include DNA synthesis, gene editing technologies like CRISPR, and computational modeling approaches to simulate and predict the behavior of engineered organisms. This approach allows researchers to precisely modify the genetic makeup of microorganisms to achieve desired characteristics.
The use of these tools can streamline the strain development process, accelerating the creation of microorganisms capable of producing complex chemicals.
Optimizing Fermentation Conditions for Enhanced Production
Fermentation conditions play a crucial role in maximizing microbial growth and product formation. Parameters like temperature, pH, aeration, and nutrient availability must be optimized to ensure optimal performance. This optimization process often involves iterative experimentation and data analysis to identify the most productive conditions.
Scaling Up Biomanufacturing Processes for Commercial Viability
Transitioning from laboratory-scale experiments to large-scale bioreactors is essential for commercial viability. This involves developing robust and cost-effective bioreactor designs, optimizing downstream processing steps for product purification and recovery, and ensuring the entire process is scalable and sustainable.
Scaling up requires careful consideration of factors such as mixing, heat transfer, and oxygen transfer within the bioreactor to maintain optimal conditions for microbial growth and product formation.
Addressing Environmental Concerns and Sustainability
The environmental impact of biomanufacturing processes must be carefully evaluated. Researchers are focusing on using renewable feedstocks and minimizing waste generation throughout the production cycle. This includes optimizing the metabolic pathways to avoid the accumulation of toxic byproducts and choosing microorganisms that can effectively utilize sustainable feedstocks.
Sustainable biomanufacturing practices are essential for creating a truly environmentally responsible and economically viable approach to chemical production.
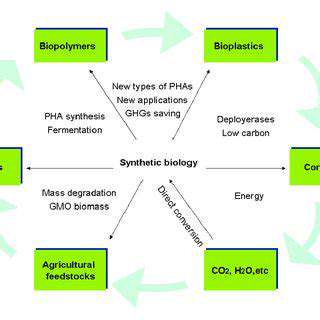
Developing Novel Bio-Based Pathways
Harnessing Microbial Metabolism
A key aspect of developing novel bio-based pathways involves harnessing the inherent metabolic capabilities of microorganisms. This approach leverages the sophisticated enzymatic machinery and metabolic networks already present within specific microbial species. By understanding and manipulating these pathways, researchers can redirect the metabolic flux towards the production of desired chemicals. This requires a deep understanding of microbial genetics and physiology, enabling targeted modifications to enhance the production efficiency and yield of the target molecule.
This process often involves identifying and optimizing key enzymes within the microbial pathway, potentially through directed evolution or rational design. Understanding the regulatory mechanisms governing metabolic fluxes is also essential. For example, manipulating gene expression to increase the production of specific enzymes involved in the synthesis of the desired molecule is a crucial step.
Designing Synthetic Gene Circuits
Synthetic biology offers powerful tools to engineer novel metabolic pathways in microorganisms. This involves designing and integrating synthetic gene circuits that precisely control the expression and activity of specific genes involved in the target chemical production. These circuits can be designed to respond to environmental cues or other stimuli, enabling more efficient and controlled production.
Crucially, the efficiency and accuracy of these circuits are vital. The design of synthetic promoters, riboswitches, and other regulatory elements is crucial to ensure the desired temporal and spatial control over gene expression, optimizing the production process.
Metabolic Engineering Strategies
Metabolic engineering strategies are crucial for optimizing bio-based pathways. These strategies involve manipulating existing metabolic pathways to enhance the production of specific chemicals, often through the introduction of new enzymes or the modification of existing ones. This can include redirecting carbon flux from central metabolic pathways to the desired product pathway, thereby improving overall efficiency.
Another important strategy involves overcoming potential bottlenecks in the metabolic pathway. Understanding the limitations of the existing pathway, such as substrate availability or enzyme kinetics, is essential for implementing appropriate engineering solutions.
Exploring Non-Canonical Pathways
Beyond modifying existing pathways, researchers are exploring non-canonical pathways, utilizing enzymes and metabolic reactions not typically found in the target organism. This requires identifying and characterizing enzymes from diverse sources, including extremophiles and other organisms with unique metabolic capabilities.
This approach can lead to the development of novel and highly efficient pathways for the production of complex chemicals that are not readily accessible through traditional metabolic engineering strategies. It also opens up the possibility of using organisms that are not traditionally used for industrial applications.
Optimization of Bioreactor Conditions
Optimizing the bioreactor conditions is crucial for maximizing the yield and efficiency of bio-based chemical production. This involves carefully controlling factors such as temperature, pH, substrate concentration, and oxygen levels. Detailed understanding and precise control of these parameters are essential to ensure optimal growth and product formation.
Furthermore, understanding the impact of different bioreactor designs on the process is crucial. This could involve exploring different mixing strategies, reactor configurations, or immobilization techniques to enhance the overall production process. Efficient bioreactor design is critical for scaling up the process to industrial levels.
Integration of Bio-based Pathways into Existing Processes
The successful implementation of bio-based pathways requires careful consideration of their integration into existing industrial processes. This includes evaluating the scalability of the process, the cost-effectiveness of the bio-based pathway, and the compatibility of the produced chemicals with downstream processes.
This step involves detailed economic analysis, process engineering, and material compatibility studies to ensure the viability and feasibility of integrating the newly developed bio-based pathways into existing industrial frameworks. Successfully integrating these pathways into existing processes is a key step towards widespread adoption and commercialization.