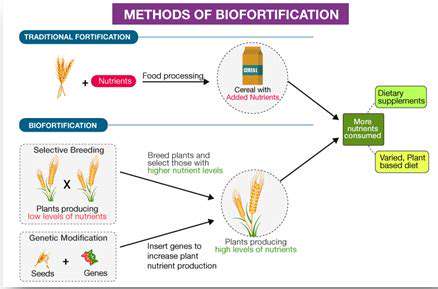
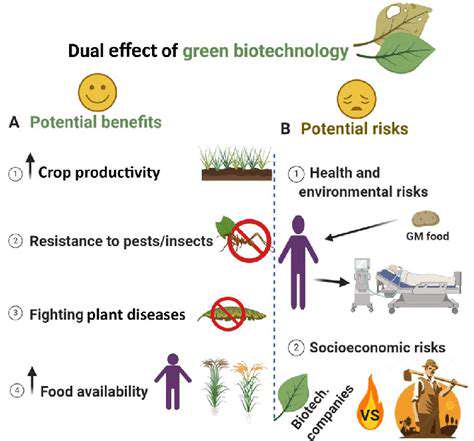
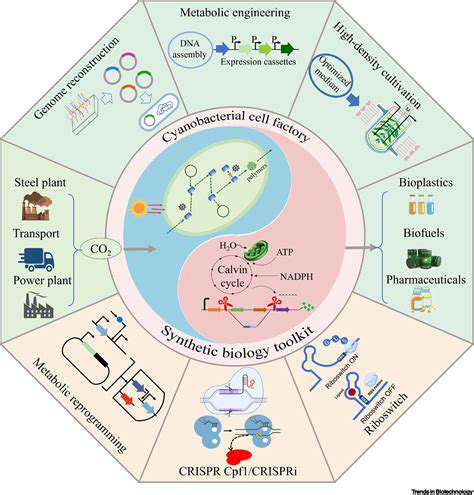
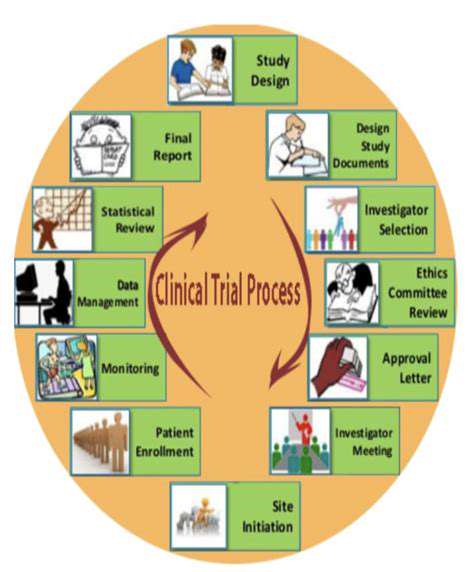
Introduction to Cell-Free Systems
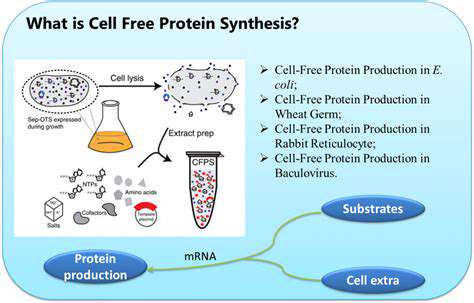
Defining Cell-Free Systems
Cell-free systems represent a groundbreaking approach in biological research, offering a powerful alternative to traditional cellular systems. These systems essentially isolate the essential components of a biological process, such as enzymes and substrates, from the complex cellular environment. This isolation allows researchers to study the process in a controlled and simplified manner, enabling a deeper understanding of its mechanistic details and potential applications. By removing the extraneous cellular machinery, researchers gain a clearer view of the specific interactions between molecules, which can be crucial for developing new therapies and biotechnological advancements.
A key advantage of cell-free systems lies in their ability to precisely control reaction conditions. This precise control enables researchers to systematically investigate the effects of different variables, including substrate concentrations, enzyme activities, and temperature, on the process being studied. This controlled environment allows for a more rigorous and reproducible study of the system's response to various stimuli, which is crucial for developing a comprehensive understanding of the system's behavior.
Applications and Advantages
The applications of cell-free systems span a wide range of biological disciplines. From fundamental research into cellular processes to the development of novel diagnostic tools and therapeutic strategies, these systems have proven invaluable in scientific exploration. They offer a unique platform to study complex biological processes, such as DNA replication, protein synthesis, and metabolic pathways, in a simplified and controllable environment. Their ability to reproduce specific biological functions in vitro provides a significant advantage over traditional cellular systems.
One particularly significant advantage of cell-free systems is their potential for high-throughput screening. These systems can be scaled up relatively easily, allowing for the rapid testing of numerous conditions and variables. This scalability, combined with their controlled environment, makes them exceptionally well-suited for drug discovery and optimization of biocatalytic processes. The ability to rapidly evaluate a large number of potential candidates for drug development or bioprocess optimization using cell-free systems is a substantial benefit over traditional approaches.
Furthermore, the modularity of cell-free systems enables the combination of different components from various sources. This allows scientists to study the interactions of these components in a controlled environment and gain insights that are difficult to obtain using traditional methods. The adaptability of cell-free systems to different research needs makes them a versatile and powerful tool in the biological research toolbox.
Cell-free systems hold great promise for future advancements in biotechnology. Their ability to precisely control reaction conditions, combined with their scalability and modularity, makes them a highly versatile platform for a wide range of applications.
Synthetic Biology's Role in CFPS Optimization
Harnessing Synthetic Biology for Enhanced CFPS Efficiency
Synthetic biology offers a powerful toolkit for optimizing cell-free protein synthesis (CFPS) systems. By engineering and modifying the components of these systems, researchers can fine-tune their performance, leading to increased protein yields and improved reaction kinetics. This involves designing and implementing new genetic circuits, optimizing enzyme activities, and developing novel strategies for substrate delivery and product recovery. This approach allows for a more targeted and controlled environment, enabling scientists to overcome limitations inherent in natural biological systems and push the boundaries of CFPS productivity.
A key aspect of this optimization involves the development of robust and efficient protein expression systems. Synthetic biology facilitates the design of optimized ribosome binding sites, promoter sequences, and terminator elements, leading to highly efficient translation of target genes. Furthermore, the incorporation of error-correcting mechanisms and codon optimization strategies can significantly reduce the occurrence of errors during protein synthesis, resulting in higher-quality protein products.
Customizing CFPS Pathways for Specific Applications
The versatility of synthetic biology extends to the customization of CFPS pathways for specific applications. Researchers can engineer these systems to produce a wide array of proteins, from therapeutic antibodies to industrial enzymes. By incorporating specific regulatory elements, researchers can control the timing and level of protein production, allowing for fine-tuning of the system for specific needs. This tailored approach is crucial for applications requiring precise control over protein synthesis, such as personalized medicine and industrial biomanufacturing.
Furthermore, synthetic biology can be leveraged to integrate CFPS systems with other biotechnologies, creating hybrid platforms for enhanced capabilities. This could involve linking CFPS with downstream processing steps, such as purification and formulation, or integrating it into microfluidic devices for miniaturized and controlled reactions. This synergistic approach aims to improve the overall efficiency and cost-effectiveness of CFPS for various applications, from basic research to industrial production.
The use of synthetic biology to create novel CFPS pathways with enhanced properties is a rapidly evolving field. The development of new strategies to optimize enzyme activity, improve substrate delivery, and enhance product recovery will likely lead to even greater improvements in CFPS efficiency in the near future. This will be pivotal in accelerating the adoption of CFPS for diverse applications ranging from drug discovery to biomanufacturing.
Further research into the use of synthetic biology for Protein engineering will likely lead to the creation of more robust and efficient CFPS pathways, further enhancing their utility across various fields. This optimization will be crucial for advancing applications such as personalized medicine and industrial biomanufacturing, where precise control over protein synthesis is essential.
Exploring the potential of synthetic biology to design and engineer specific CFPS systems tailored to unique needs will revolutionize how we approach protein production. This approach could lead to significant improvements in yield, purity, and cost-effectiveness, making CFPS a more attractive and accessible technology for a wider range of applications.
Advanced Applications in Drug Discovery and Development
Harnessing Synthetic Biology for Targeted Drug Delivery
Synthetic biology offers exciting avenues for developing novel drug delivery systems. By engineering cells to express proteins that respond to specific stimuli, researchers can create targeted drug carriers capable of delivering therapeutic agents directly to diseased tissues. This precise delivery method minimizes systemic side effects, maximizing the efficacy of the treatment while reducing potential harm to healthy organs. The potential for creating biocompatible, self-assembling drug delivery systems opens up new possibilities in personalized medicine.
Imagine a scenario where immune cells are engineered to recognize and destroy cancer cells, carrying potent anti-cancer drugs directly to the tumor site. This approach holds immense promise for treating a wide range of diseases, from cancer to infectious diseases, with far greater precision and fewer side effects than traditional methods.
Designing Novel Drug Candidates Using Synthetic Biology
Synthetic biology enables the creation of entirely new biological systems to produce novel drug candidates. By modifying existing pathways or introducing entirely new ones, researchers can engineer microorganisms to produce small molecule drugs, proteins, or even complex biological systems for therapeutic applications. This approach offers the potential to produce a wide array of molecules with unique properties and specific targeting mechanisms.
This innovative approach allows for the rapid and cost-effective production of drug candidates that may be difficult or impossible to obtain through traditional methods. The potential to tailor the characteristics of these molecules to specific diseases promises to revolutionize drug discovery and development.
Accelerating Drug Screening and Testing
Synthetic biology can significantly accelerate the drug screening process. By creating cell lines or organisms that mimic specific aspects of human biology, researchers can conduct high-throughput screening of potential drug candidates with greater accuracy and efficiency. This speeds up the identification of promising compounds, reducing the time and resources required for traditional drug discovery methods. Advanced biological systems can be engineered to identify and quantify specific molecular interactions with greater precision, leading to faster and more accurate results.
Creating Personalized Therapies through Synthetic Biology
The ability to engineer cells to respond to specific genetic or environmental cues paves the way for personalized medicine. Synthetic biology can be used to create customized therapies based on an individual's genetic makeup, enabling the development of treatments tailored to specific patient needs. This approach offers the potential to develop more effective and safer therapies while minimizing adverse side effects.
Improving Diagnostics through Synthetic Biology Tools
Synthetic biology is revolutionizing diagnostic tools, enabling the development of more sensitive and specific diagnostic platforms. By engineering biosensors or bio-indicators, researchers can detect disease markers or pathogens with greater accuracy. These biosensors can be designed to respond to specific molecular targets, enabling early and precise diagnoses, potentially leading to quicker interventions and improved patient outcomes.
Developing Sustainable and Cost-Effective Drug Manufacturing
Synthetic biology offers the potential to create more sustainable and cost-effective methods for drug manufacturing. Engineering microorganisms to produce therapeutic molecules can reduce the reliance on traditional chemical synthesis, minimizing environmental impact and lowering production costs. This approach can also enable the production of drugs in remote or resource-limited settings, making them more accessible to patients worldwide. The ability to control and optimize biological processes for drug production promises to revolutionize pharmaceutical manufacturing.
Tailoring Protein Properties with Synthetic Biology
Protein Engineering for Enhanced Functionality
Protein engineering, a powerful tool in biotechnology, allows scientists to modify existing proteins or design entirely new ones with specific functionalities. This process involves manipulating the amino acid sequence of a protein, leading to alterations in its structure and consequently, its properties. By carefully selecting and modifying amino acid residues, researchers can optimize proteins for diverse applications, from pharmaceuticals to industrial processes. This meticulous tailoring process enables the creation of proteins with enhanced stability, improved activity, and increased selectivity, thus unlocking novel possibilities in various fields.
One of the key applications of protein engineering is the development of more effective therapeutic agents. Modifying proteins involved in disease processes can lead to drugs with higher efficacy and reduced side effects. For instance, engineers can enhance the binding affinity of antibodies to target antigens, increasing the effectiveness of immunotherapy. This approach also holds promise for creating more stable enzymes for industrial applications.
Design Considerations for Protein Modification
Several crucial factors are considered when designing protein modifications. Understanding the relationship between protein structure and function is paramount. Precise amino acid substitutions are carefully chosen to achieve the desired alteration in properties. Computational modeling plays a significant role in predicting the impact of these changes on protein stability and activity. This iterative process of design, testing, and refinement is essential for optimizing the protein's final functionality.
Factors such as the protein's three-dimensional structure, its interaction partners, and its specific environment are carefully considered. Scientists must also account for potential side effects of the modifications, ensuring that the engineered protein retains its desired properties while minimizing any unwanted consequences. This comprehensive understanding is crucial for creating effective and safe protein-based products.
Applications and Impacts of Tailored Proteins
The applications of tailored proteins are vast and span numerous fields. In the pharmaceutical industry, engineered proteins are used to develop novel drugs, diagnostics, and vaccines. These advancements promise to revolutionize disease treatment and prevention. In the food industry, protein engineering can create enzymes with improved efficiency in food processing, enhancing nutritional value and extending shelf life. Furthermore, in industrial settings, tailored proteins can be used as catalysts in various processes, leading to greater efficiency and reduced environmental impact.
The impact of protein engineering extends beyond these specific examples. It fosters innovation, driving advancements in diverse fields. This innovative approach promises to bring about significant breakthroughs in areas like healthcare, agriculture, and environmental science. It underscores the potential of biotechnology to tackle global challenges and improve the quality of life for people worldwide.
The Future of CFPS and Synthetic Biology Integration
Harnessing the Power of CFPS for Enhanced Synthetic Biology Design
CFPS, or cell-free protein synthesis, offers a powerful platform for synthetic biology applications. Its inherent flexibility and scalability make it an attractive alternative to traditional cellular systems. By decoupling protein synthesis from the complex regulatory networks of living cells, CFPS allows for greater control over the production of desired proteins, enabling researchers to optimize reaction conditions and streamline the design process for novel biomolecules and pathways. This control extends to the manipulation of reaction rates, substrate concentrations, and enzyme activity, providing a more predictable and efficient synthetic biology workflow compared to traditional cellular systems.
Streamlining Protein Production and Optimization with CFPS
One of the key advantages of integrating CFPS into synthetic biology is the ability to streamline protein production. Traditional methods often involve complex cellular processes and lengthy optimization steps. CFPS, on the other hand, offers a more defined and controllable environment. This translates to faster protein production cycles and reduced costs, particularly for high-throughput screening and the rapid generation of protein variants.
Furthermore, the precise control over reaction parameters in CFPS allows for optimization of protein expression and purification. Researchers can meticulously adjust factors like temperature, pH, and substrate concentrations to achieve optimal protein yields and purity, directly influencing the efficiency of downstream applications.
Addressing Challenges in Synthetic Biology with CFPS
While CFPS presents numerous opportunities for synthetic biology, there are also challenges to consider. One hurdle is the potential for limitations in the complexity of the biological systems that can be engineered within a cell-free platform. However, ongoing research is actively addressing these limitations by developing new strategies for incorporating complex genetic circuits and regulatory networks within CFPS systems. Developing robust and scalable methods for incorporating and maintaining these systems is crucial for broader implementation in synthetic biology.
Exploring Applications in Diverse Fields
The integration of CFPS with synthetic biology has wide-ranging applications across numerous scientific disciplines. In biomanufacturing, CFPS can be used to produce therapeutic proteins, enzymes, and other valuable biomolecules with higher efficiency and lower costs. In agriculture, CFPS could be instrumental in developing novel biofertilizers and biopesticides, while in environmental science, it could contribute to the development of bioremediation strategies. The possibilities are extensive and continue to expand as researchers explore new avenues for combining these powerful technologies.
Future Directions and Research Priorities
The future of CFPS and synthetic biology integration hinges on continued research and development. Key areas of focus include enhancing the scalability and cost-effectiveness of CFPS systems, improving the design and implementation of complex genetic circuits within these platforms, and exploring novel applications in diverse fields. Collaboration between synthetic biologists, biochemists, and engineers is essential to overcome current challenges and fully realize the potential of this powerful technology.