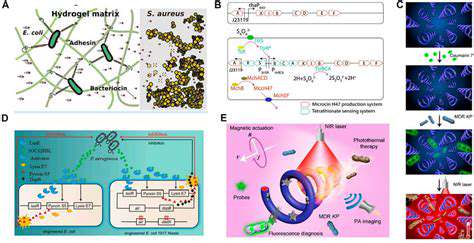
Improving Cellular Efficiency and Stability
Long-term production viability depends on maintaining robust microbial strains. Researchers address this through various strategies: enhancing plasmid stability, optimizing growth conditions, and implementing evolutionary pressure techniques to preserve desired traits. These measures ensure consistent performance across generations of cell division, crucial for industrial-scale operations.
Addressing Challenges in Scale-Up and Cost-Effectiveness
Transitioning from lab-scale to commercial production presents unique hurdles. Engineers must design bioreactor systems that maintain optimal conditions while accommodating massive cell populations. Process intensification strategies, such as continuous fermentation and in-situ product removal, can dramatically improve throughput and reduce per-unit costs.
Ethical Considerations and Future Directions
As with any emerging technology, responsible development is paramount. Rigorous containment protocols and environmental impact assessments must accompany industrial implementation. The field continues to evolve with promising avenues like cell-free biosynthesis systems and AI-driven pathway design, pointing toward even more sustainable pharmaceutical manufacturing in the future.
Optimizing Microbial Hosts for Enhanced Production Efficiency
Improving Metabolic Pathways for Enhanced Productivity
Microbial optimization begins with metabolic rewiring. By applying systems biology approaches, researchers can identify key control points in cellular metabolism. Strategic modifications might include introducing heterologous enzymes or creating synthetic regulatory circuits to precisely control flux through desired pathways. This fine-tuning maximizes carbon conversion efficiency while minimizing metabolic burden.
Feedstock flexibility represents another important frontier. Engineering microbes to utilize alternative carbon sources - from agricultural waste to industrial byproducts - could significantly reduce production costs. Such advancements align with growing demands for sustainable biomanufacturing practices across the pharmaceutical sector.
Enhancing Cellular Machinery and Stress Tolerance
Beyond metabolic engineering, attention must focus on cellular infrastructure. Modifications to protein synthesis machinery, chaperone systems, and secretion pathways can dramatically improve production capacity. Implementing stress-responsive genetic circuits helps maintain performance under industrial conditions, ensuring reliable operation despite fluctuations in temperature, pH, or nutrient availability.
Designing Novel Metabolic Pathways for Biopharmaceutical Synthesis
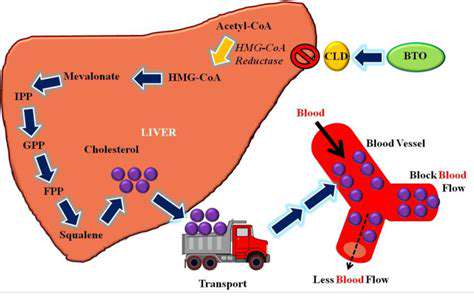
Harnessing Nature's Blueprint
Biological systems have evolved remarkable chemical factories over billions of years. Modern researchers now combine this natural inventory with computational tools to design bespoke metabolic routes for pharmaceutical synthesis. This hybrid approach blends evolutionary wisdom with human ingenuity to create pathways that might never emerge in nature.
Enzymatic Catalysts: The Workhorses of Pathways
Enzyme engineering plays a pivotal role in pathway design. Through directed evolution and rational design, scientists can reshape catalytic properties to suit specific industrial needs. This might involve improving substrate specificity, altering cofactor requirements, or enhancing stability under process conditions.
Computational Modeling and Pathway Design
Advanced simulation tools now allow researchers to virtually prototype metabolic networks before laboratory implementation. These models can predict flux distributions, identify potential bottlenecks, and suggest optimization strategies. In silico testing significantly accelerates the design-build-test cycle, reducing development timelines and costs.
The era of personalized medicine demands more flexible production systems. Microbial factories offer inherent advantages here, as genetic reprogramming allows relatively quick adaptation to produce different therapeutic compounds. This versatility aligns well with the need for patient-specific treatments and niche-market pharmaceuticals.
Improving Bioreactor Design for Enhanced Productivity
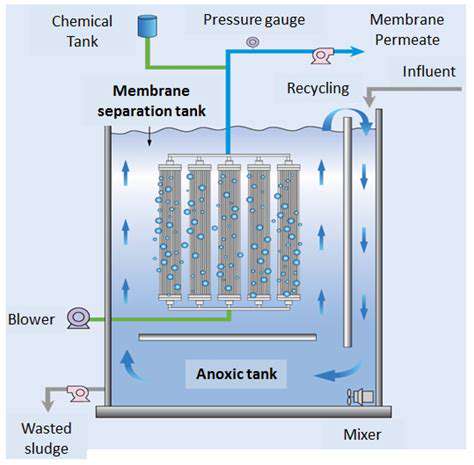
Optimizing Nutrient Delivery
Modern bioreactor engineering focuses on creating precisely controlled microenvironments. Advanced sensor arrays coupled with machine learning algorithms enable real-time adjustment of feeding strategies. This dynamic approach prevents nutrient limitation while avoiding wasteful excess, maximizing both cell growth and product formation.
Enhancing Mass Transfer
Gas exchange presents particular challenges at industrial scales. Novel impeller designs and microbubble aeration systems can dramatically improve oxygen transfer rates. These innovations help maintain aerobic conditions even in dense cell cultures, preventing oxygen limitation that could compromise product yields.
Addressing Challenges and Future Directions

Navigating the Complexities of Data Integration
Process optimization requires synthesizing data from multiple analytical platforms. Implementing unified data architectures allows researchers to correlate genetic modifications with phenotypic outcomes and production metrics. This systems-level understanding is crucial for continuous improvement of microbial strains and bioprocesses.
Leveraging Emerging Technologies for Data Analysis
Machine learning algorithms are proving invaluable for pattern recognition in complex biological datasets. These tools can identify non-obvious correlations between genetic modifications and production outcomes, suggesting new avenues for strain improvement that might elude human researchers.