Specific Outsourcing Areas in Drug Development

Pharmaceutical Manufacturing
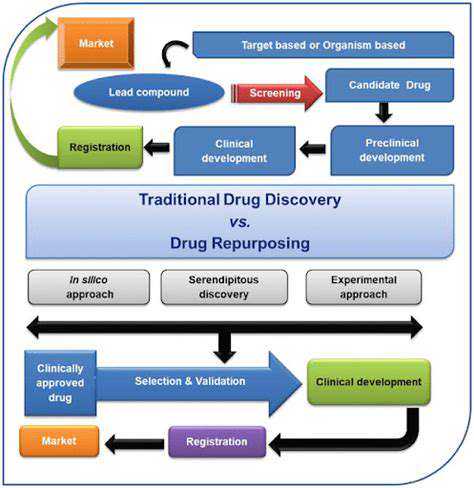
Outsourcing pharmaceutical manufacturing offers significant benefits, allowing companies to focus on core competencies like research and development while leveraging specialized facilities and expertise. This specialized expertise often includes stringent quality control and regulatory compliance, crucial for maintaining product safety and efficacy. Outsourcing manufacturing can also reduce capital expenditures and operational costs associated with setting up and maintaining a dedicated facility.
Companies can select manufacturers based on their specific needs, choosing facilities with expertise in particular drug forms (e.g., tablets, injectables, or sterile products). This tailored approach ensures the manufacturing process aligns with the desired quality and regulatory standards. This strategic approach can significantly impact the overall cost of goods sold, making it an attractive option for many pharmaceutical companies.
Clinical Research and Trials
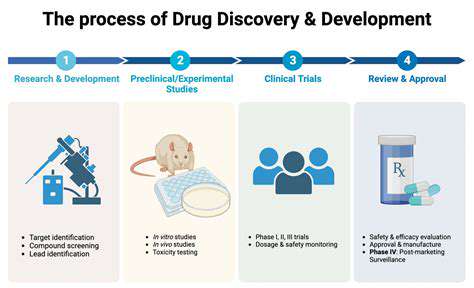
Outsourcing clinical research and trials is a crucial aspect of bringing new drugs to market. Experienced CROs (Contract Research Organizations) handle the entire process, from patient recruitment to data analysis, freeing up internal resources. This allows companies to concentrate on drug development and regulatory submissions. Outsourcing these trials often reduces the time to market for new drugs.
Outsourcing clinical trials can also provide access to a broader patient pool and specialized expertise in various therapeutic areas, leading to more robust and reliable data. This can expedite the drug development process and increase the chances of regulatory approval. Furthermore, CROs often have established relationships with regulatory bodies, streamlining the entire process.
Regulatory Affairs
Handling regulatory affairs can be a complex and time-consuming process for pharmaceutical companies. Outsourcing this function to specialized regulatory affairs consultants can significantly reduce the burden on internal teams. These consultants possess extensive knowledge of regulatory guidelines and procedures, ensuring compliance with all applicable regulations.
This expertise is essential for navigating the intricacies of drug approval processes in different countries and regions. The consultants can help companies prepare comprehensive regulatory documents, streamline submissions, and manage interactions with regulatory authorities. This frees internal resources to focus on other critical aspects of the business.
Formulation Development
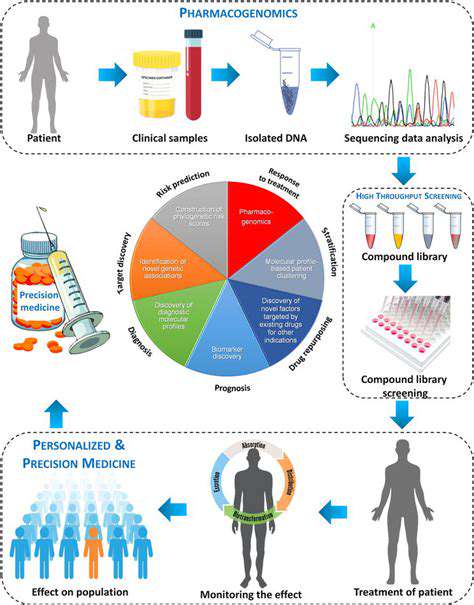
Outsourcing formulation development is a valuable strategy for pharmaceutical companies seeking to optimize drug properties. External experts can conduct research and experiments to develop new and improved formulations, considering factors like bioavailability, stability, and patient tolerability. This expertise can lead to innovative drug delivery systems, improving therapeutic outcomes and patient adherence.
Outsourcing often provides access to advanced equipment and technologies that may not be readily available in-house. This access can help develop novel formulations with enhanced therapeutic benefits and potentially more efficient manufacturing processes. This can be a significant advantage in bringing innovative drugs to the market.
Packaging and Labeling
Outsourcing packaging and labeling services is an important part of the supply chain for pharmaceutical products. This aspect of manufacturing involves expertise in creating appropriate packaging to maintain the integrity of the drug product. Companies can leverage specialized packaging solutions to optimize storage and transport of the drugs, ensuring their safety and efficacy.
Experienced providers also handle regulatory requirements for labeling, ensuring compliance with all relevant guidelines. This includes designing labels that are informative, accurate, and compliant with labeling standards. Furthermore, outsourcing these services can improve efficiency and reduce costs, freeing up internal resources for other critical tasks.
Quality Control and Assurance
Maintaining strict quality control and assurance is paramount in the pharmaceutical industry. Outsourcing these critical functions to dedicated quality assurance providers can enhance the overall quality and safety of the final product. These providers typically have robust quality management systems in place, ensuring rigorous testing and validation procedures.
Outsourcing quality control enables the company to leverage specialized expertise in various testing methods and analytical techniques. This allows for thorough evaluation of the drug product at different stages of manufacturing, from raw materials to finished goods. This focus on quality and assurance ensures that the final product meets the highest standards of safety and efficacy.