Current Research and Clinical Trials

Current Research Directions
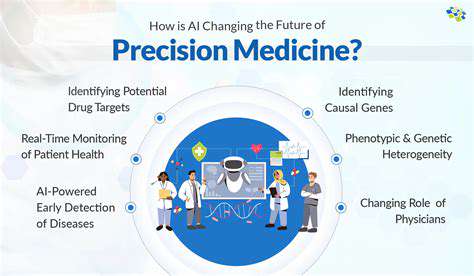
Scientists are now prioritizing customized methods for managing diseases, shifting away from one-size-fits-all solutions. Cutting-edge tools like genomics and proteomics help pinpoint unique biomarkers that forecast how patients might react to treatments, enabling therapies to be fine-tuned for each individual. This shift toward precision medicine could dramatically enhance treatment success rates while lowering harmful side effects.
Clinical Trial Design Innovations
Modern clinical trials are adopting flexible designs that adjust as new data emerges. These adaptive approaches let researchers tweak study parameters mid-trial, speeding up the discovery of effective treatments while sparing participants from ineffective ones. Such flexibility proves invaluable for optimizing research efficiency.
Pharmacogenomics in Clinical Trials
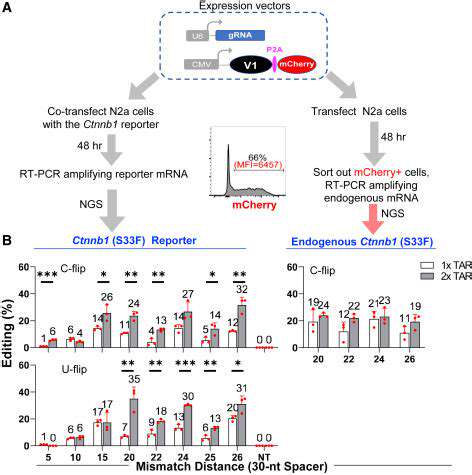
Genetic insights are revolutionizing how clinical trials are conducted. By analyzing patients' DNA, scientists can predict medication responses with greater accuracy. This genetic matching helps identify ideal candidates for specific treatments while minimizing dangerous drug interactions. Understanding how genes affect drug processing has become fundamental to modern trial design.
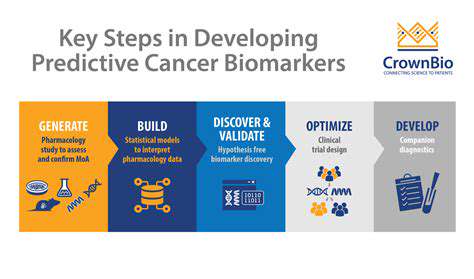
Biomarkers for Early Disease Detection
Discovering reliable early warning signs for diseases dominates current research efforts. Catching illnesses sooner means interventions can begin earlier, potentially slowing disease advancement. Investigators are examining multiple detection methods, from simple blood work to advanced imaging and genetic screening. Such breakthroughs could transform healthcare by enabling proactive treatment before conditions worsen.
Data Analysis and Interpretation
Revolutionary data processing techniques are reshaping clinical research. Powerful statistical models and AI-driven analysis uncover hidden patterns in massive datasets. This analytical leap forward provides deeper understanding of diseases and treatment effectiveness. Enhanced data interpretation also fuels more precise forecasting of patient results.
Ethical Considerations in Clinical Trials
Moral responsibility remains central to medical research. Protecting participants' wellbeing, privacy, and right to make informed choices forms the ethical foundation of all studies. Strict adherence to ethical codes ensures volunteer safety from start to finish. Maintaining transparency throughout the research process also builds essential public trust.
Regulatory Pathways for New Treatments
Approval processes for novel therapies grow increasingly intricate. While thorough safety evaluations remain crucial, there's growing demand for expedited review systems. Regulators face the delicate challenge of accelerating access to breakthrough treatments without compromising safety standards. Finding this balance ensures patients benefit from innovations while being protected from potential risks.

Ethical Considerations and Public Perception
Ethical Implications of Germline Editing
Altering inheritable genetic material sparks intense ethical debate. The specter of unintended consequences – both foreseen and unforeseen – looms large over this technology. While eliminating genetic blindness offers hope, the risk of introducing new health complications for descendants demands careful long-term study. Society must engage in inclusive discussions about genetic modification boundaries, involving experts from multiple disciplines alongside community representatives.
The technology's potential for misuse and exacerbating social inequalities presents another ethical minefield. Without proper safeguards, gene editing could become a privilege of the wealthy, creating genetic disparities. Fair access policies and responsible governance must ensure these medical advances benefit everyone equally.
Public Perception and Societal Impact
Community attitudes toward vision-correcting gene therapies will significantly influence their adoption. Misinformation and fears about genetic manipulation could stall progress. Transparent communication about both the capabilities and limitations of this technology proves essential for building public confidence.
Comprehensive education initiatives must address public concerns while accurately presenting the science. These programs should emphasize the life-changing potential for blindness sufferers while candidly addressing the complex moral questions involved.
Informed Consent and Patient Autonomy
Patients considering genetic treatments deserve complete information about possible outcomes. Truly informed consent requires understanding both the potential vision improvements and possible complications, including the need for lifelong monitoring. Decision-making power must remain firmly with patients after full disclosure of all relevant factors.
Societal Responsibility and Governance
Developing strong international standards for gene editing research and application remains critical. Collaborative global oversight can help ensure responsible development of vision restoration techniques. Clear guidelines must define acceptable genetic modifications and establish monitoring systems to prevent misuse.
Long-Term Monitoring and Follow-up
Extended patient tracking represents a non-negotiable component of gene therapy. Researchers must document both immediate results and delayed effects, tracking vision improvements and potential complications over decades. Only through thorough, ongoing evaluation can we guarantee these treatments remain safe throughout patients' lives.
Accessibility and Equity of Care
Making genetic vision therapies available to all who need them constitutes a moral obligation. The high costs of these advanced procedures must be addressed through innovative funding models and policy solutions. Geographic and economic barriers shouldn't prevent anyone from benefiting from these medical breakthroughs.
Potential for Off-Target Effects
While promising, gene editing carries inherent risks of unintended genetic changes. Strict laboratory protocols and thorough safety testing help minimize these accidental modifications. Robust systems for identifying and addressing any off-target effects must be implemented to protect patients undergoing treatment.