Beyond the Cas9 Revolution: Exploring Base Editing
Base editing technology represents a significant evolution in genetic engineering, offering potential advantages over traditional CRISPR-Cas9 approaches. By directly converting one DNA base to another without double-strand breaks, this method may reduce unwanted genetic alterations. The technology shows particular promise for correcting point mutations associated with numerous genetic disorders.
Recent advances in base editor design have significantly improved their precision and versatility. These refinements address some limitations of earlier gene editing approaches while opening new possibilities for therapeutic applications.
Prime Editing: A New Era of Precision Gene Editing
Prime editing stands as a major technological leap forward in genome engineering. This innovative approach combines the targeting precision of CRISPR with novel editing mechanisms that avoid DNA breaks. The system's ability to make precise insertions, deletions, and base conversions expands the range of possible genetic corrections.
The versatility of prime editing suggests it may become a valuable tool for addressing complex genetic defects that were previously difficult or impossible to correct using existing technologies.
Transcription Activator-Like Effector Nucleases (TALENs): A Pioneering Approach
TALEN technology represents an important chapter in the history of genome engineering. These customizable proteins demonstrated the feasibility of targeted genetic modifications before the CRISPR revolution. The modular design of TALENs allows for precise targeting of specific DNA sequences, maintaining their relevance in certain research applications.
While largely supplanted by newer technologies, TALENs continue to provide valuable insights into the fundamental principles of targeted genome editing and its therapeutic potential.
Zinc Finger Nucleases (ZFNs): Early Pioneers in Gene Editing
ZFNs were instrumental in establishing the field of targeted genome editing. These engineered proteins demonstrated that specific DNA sequences could be precisely targeted and modified. While their complexity limited widespread adoption, ZFNs provided critical proof-of-concept for subsequent gene editing technologies.
The development of ZFN technology laid essential groundwork for understanding the challenges and possibilities of targeted genetic modifications in therapeutic contexts.
Meganucleases: Targeting Large DNA Sequences with Precision
Meganuclease technology offers unique advantages for certain genetic engineering applications. Their ability to recognize and cleave longer DNA sequences enables precise editing of larger genomic regions. This capability may prove valuable for addressing complex genetic modifications that require more extensive changes to the DNA sequence.
RNA-Guided Nucleases Beyond CRISPR: Exploring Novel Approaches
The field continues to explore alternative RNA-guided nuclease systems that may offer complementary advantages to CRISPR-based tools. These emerging technologies may provide solutions to specific challenges in gene editing, such as improved specificity or novel delivery mechanisms. The diversity of these systems suggests a rich future for genome engineering technologies.
Addressing Challenges and Future Directions
Improving Gene Editing Techniques for Enhanced Efficiency
Current gene editing platforms show tremendous potential but require continued refinement. Researchers are working to develop systems capable of targeting multiple genetic elements simultaneously, which could significantly improve therapeutic outcomes. Optimizing delivery methods remains a critical focus, with particular emphasis on achieving cell-type specificity.
The development of advanced delivery systems, including targeted nanoparticles, may help address current limitations in getting editing tools to the right cells while minimizing effects on healthy tissue.
Targeting Viral Replication Mechanisms
A comprehensive understanding of viral life cycles is essential for developing effective genetic interventions. Identifying critical viral dependencies provides opportunities for targeted disruption using gene editing technologies. This approach may lead to novel strategies for preventing viral replication and spread.
Research into viral-host interactions continues to reveal new targets for therapeutic intervention, potentially leading to more effective treatments for viral infections.
Developing Multi-pronged Strategies
Combining gene editing with existing antiviral approaches may create synergistic effects that improve treatment outcomes. Integrated strategies that target multiple aspects of viral infection could provide more robust protection against evolving pathogens.
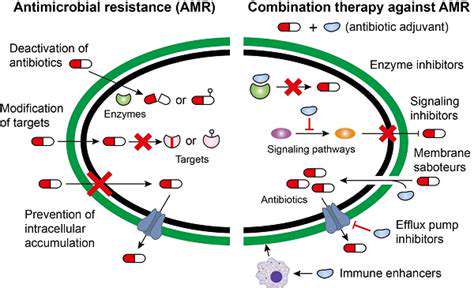
Addressing Viral Resistance
The potential for viral resistance to gene editing interventions requires careful consideration. Developing strategies that target multiple viral components simultaneously may help prevent the emergence of resistant strains. Long-term monitoring will be essential to understand the durability of gene editing-based antiviral approaches.
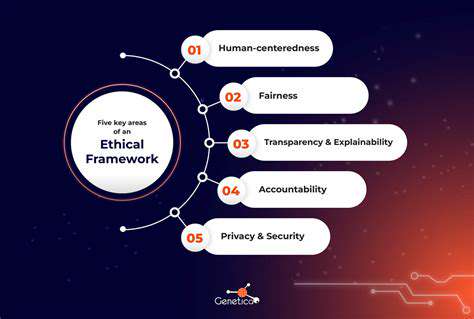
Ethical Considerations and Public Health Implications
The application of gene editing technologies to viral infections raises important ethical questions that merit careful discussion. Issues of safety, access, and equitable distribution require thoughtful consideration as these technologies advance. Public engagement and transparent dialogue will be crucial for responsible development of these powerful tools.
Translational Research and Clinical Trials
Moving gene editing therapies from bench to bedside requires rigorous clinical evaluation. Careful study design and thorough safety assessments will be essential to establish the efficacy and safety of these novel interventions. The field continues to develop standards and best practices for clinical translation of gene editing technologies.