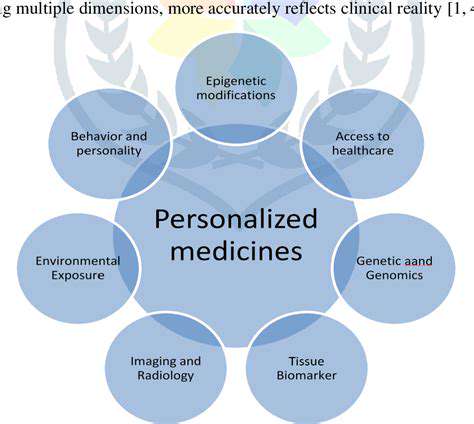
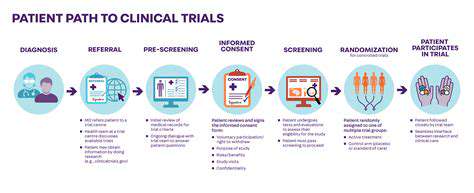
Phase III Trial Design and Methodology
Phase III trials represent a crucial step in the drug development process, designed to confirm the efficacy and safety of a treatment in a larger, more diverse population. These trials typically involve a significant number of participants, often thousands, and are rigorously designed to minimize bias and maximize the reliability of the results. Careful consideration of the inclusion and exclusion criteria is paramount, ensuring the study population accurately reflects the target patient group. This meticulous design allows researchers to assess the treatment's effectiveness under real-world conditions and to identify potential adverse effects that might not have been apparent in earlier phases.
The methodology employed in Phase III trials is highly standardized, adhering to strict ethical guidelines and regulatory requirements. This ensures data integrity and allows for comparisons across different studies. Blinding techniques, where participants and researchers are unaware of the treatment assignment, are often employed to minimize bias in both outcome assessment and participant behavior. This crucial element helps to isolate the effect of the treatment itself, separating it from potential placebo effects or other confounding variables. Statistical analyses of the collected data are meticulously performed to determine the significance of the observed effects, providing evidence for or against the treatment's efficacy.
Key Considerations for Phase III Trials
One of the most critical considerations in Phase III trials is the appropriate sample size calculation. This calculation ensures the study has sufficient power to detect a clinically meaningful difference between the treatment and control groups, if one exists. A precise and well-reasoned sample size calculation is essential to avoid unnecessary costs and participant burden, while still maintaining the statistical rigor required for robust conclusions.
Safety monitoring is another critical aspect of Phase III trials. Throughout the trial, safety data are continuously collected and analyzed to identify any adverse events, regardless of their apparent severity. This proactive monitoring allows for prompt intervention if necessary to protect participant well-being and to gain a comprehensive understanding of the treatment's safety profile. Close collaboration between researchers, regulatory bodies, and ethics committees is essential throughout the trial process to ensure the highest standards of ethical conduct.
The duration of Phase III trials can vary considerably depending on the specific treatment and the characteristics of the target population. Careful consideration must be given to the length of time needed to observe the treatment's effects and to collect sufficient data for accurate analysis. This necessitates meticulous planning and resource allocation to ensure that the trial is completed effectively and efficiently. The overall goal is to gather enough robust data to support a regulatory submission for the treatment's approval.
Phase IV Trials: Post-Market Surveillance and Long-Term Effects
Understanding Phase IV Trials
Phase IV clinical trials, also known as post-market surveillance studies, are a crucial component of the drug development process. These trials occur after a drug has been approved and released for general use. Their primary goal is to gather further information about the drug's long-term effects, identify rare side effects, and optimize its use in various patient populations. This ongoing monitoring helps refine treatment strategies and ensure the drug's safety and effectiveness over time.
Unlike earlier phases, which focus on establishing safety and efficacy in specific populations, Phase IV trials often involve a broader range of patients, potentially uncovering side effects or interactions that weren't apparent in the earlier, smaller studies. This expanded view of the drug's impact is vital for informing healthcare practitioners and patients about potential risks and benefits.
Post-Market Surveillance: Why is it Necessary?
Post-market surveillance is essential because drug effects can sometimes manifest only after prolonged use. Rare side effects, interactions with other medications, or long-term consequences may not be detectable during earlier phases, which typically involve smaller and more homogenous patient groups. The increased patient population in Phase IV allows researchers to observe a wider range of reactions and potential problems.
Furthermore, as medical knowledge evolves and our understanding of diseases deepens, Phase IV trials can help refine the use of a medication. New information might emerge that suggests adjustments to dosage, patient selection, or combination therapies to maximize benefits and minimize risks.
Methods and Data Collection in Phase IV Trials
Phase IV trials employ various methodologies to collect data. Researchers often use observational studies, where they monitor patients who are already taking the drug. This can involve gathering data from electronic health records, conducting questionnaires, or performing follow-up assessments. The focus is on gathering real-world data about the drug's impact in diverse patient settings, which can lead to a more comprehensive understanding of its effects.
Sometimes, randomized controlled trials (RCTs) are conducted during Phase IV. These trials compare the drug to existing treatments or placebos to further assess its effectiveness and safety in specific contexts. These different methods provide a robust picture of the drug's long-term profile.
Long-Term Effects and Safety Monitoring
Long-term effects are a critical focus of Phase IV trials. Researchers diligently monitor patients for any adverse events that may emerge over extended periods. This includes monitoring for rare side effects, cumulative toxicity, and the development of new complications. Understanding the long-term safety profile is paramount for ensuring the continued safe use of the drug and for informing appropriate patient management.
The data gathered in these trials is often utilized to update prescribing information and patient education materials, helping to ensure that healthcare professionals and patients are well-informed about the drug's potential risks and benefits.
The Role of Patient Participation in Phase IV Trials
Patient participation is crucial in Phase IV trials. Patients who volunteer to participate provide critical insights into their experiences with the drug, helping to identify potential issues and refine treatment strategies. Their feedback and perspectives are invaluable in ensuring the drug's continued safe and effective use. Patient engagement also fosters trust and transparency in the drug development process.
Ethical Considerations in Phase IV Trials
Ethical considerations are paramount in Phase IV trials. Researchers must ensure patient confidentiality, informed consent, and equitable access to the necessary monitoring and support. A rigorous ethical review process is essential to minimize risks and ensure that the trial is conducted in a responsible and ethical manner. Transparency and open communication with patients are key elements to maintain public trust.
Maintaining the integrity of the data collected is also critical. Strict adherence to data handling protocols and rigorous quality control measures must be in place to ensure the reliability and validity of the results from Phase IV trials.