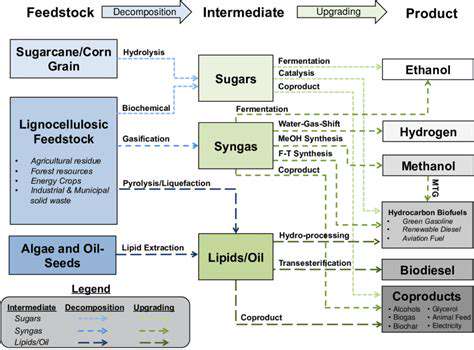
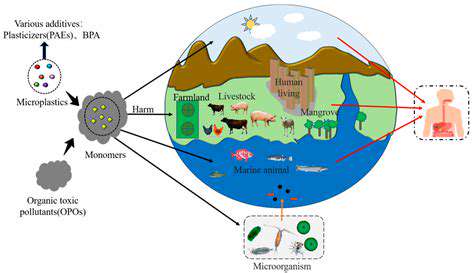
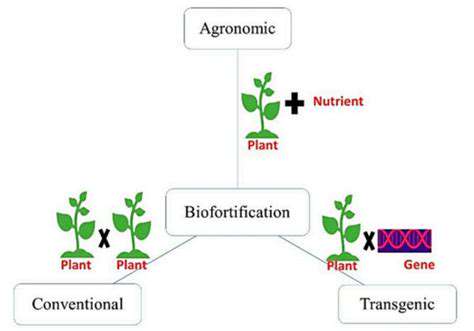
Personalized medicine represents a paradigm shift in healthcare, moving away from a one-size-fits-all approach to treatment. By analyzing individual genetic, environmental, and lifestyle factors, clinicians can tailor therapies to optimize efficacy and minimize adverse effects. This approach promises to improve patient outcomes by identifying the most effective treatments for specific individuals, ultimately leading to better health and reduced healthcare costs.
This individualized approach considers the unique characteristics of each patient, which can vary significantly. Genetic variations, for example, can influence how a person metabolizes medications, impacting treatment response. Understanding these individual differences is crucial for selecting the most appropriate and effective therapies.
Targeted Therapies: Focusing on Specific Molecular Targets
Targeted therapies represent a crucial component of personalized medicine. These therapies are designed to specifically interfere with the growth and spread of cancer cells by targeting specific molecules or pathways involved in their development and progression. This precision approach aims to minimize damage to healthy cells, thereby reducing side effects and improving treatment outcomes.
Through the identification of specific molecular targets, targeted therapies can effectively destroy or inhibit the growth of cancer cells without harming healthy tissues. This approach has revolutionized cancer treatment, offering hope for improved survival rates and quality of life for patients.
The Role of Genomics in Personalized Medicine
Genomics plays a pivotal role in personalized medicine by enabling the analysis of an individual's complete set of genes. This information helps to identify genetic variations that may influence drug response, susceptibility to diseases, and predisposition to certain conditions. By understanding an individual's genetic makeup, clinicians can make more informed decisions about treatment plans and preventive measures.
Digital Health Tools for Treatment Monitoring
Digital health tools, such as wearable devices and mobile applications, facilitate continuous monitoring of patients' health status. This continuous data collection allows clinicians to track treatment effectiveness, identify potential adverse reactions, and adjust therapies as needed. These tools promote proactive healthcare, empowering patients to take an active role in their own health management.
Pharmacogenomics: Understanding Drug Response
Pharmacogenomics is a branch of genomics that focuses on the relationship between an individual's genetic makeup and their response to medications. By analyzing specific genes, pharmacogenomics can predict how a person will metabolize and respond to a particular drug. This information is invaluable for selecting the optimal drug dosage and type, minimizing adverse reactions, and maximizing treatment efficacy.
Big Data Analytics for Precision Treatment
Big data analytics has emerged as a powerful tool for analyzing vast amounts of patient data, including genomic information, medical history, and lifestyle factors. This analysis enables the identification of patterns and correlations that can guide the development of personalized treatment strategies. The ability to process and interpret large datasets allows for the identification of nuanced relationships and the creation of more accurate predictive models for treatment outcomes.
Ethical Considerations of Personalized Medicine
The implementation of personalized medicine raises several important ethical considerations, including data privacy, access to technology, and equitable distribution of resources. Careful consideration must be given to ensuring that sensitive patient data is protected, that access to these advanced therapies is not limited by socioeconomic status, and that the benefits of personalized medicine are distributed fairly across all populations. Addressing these ethical concerns is paramount for responsible implementation and societal acceptance of this transformative approach to healthcare.
Streamlined Clinical Trials and Regulatory Processes
Improving Trial Efficiency
Digital tools can significantly accelerate clinical trial timelines by automating data collection, reducing administrative burdens, and enabling remote patient monitoring. This streamlined process not only saves time and resources but also allows researchers to gather more comprehensive data, leading to more robust and reliable results. The potential for faster drug development and approval translates directly into faster access to life-saving treatments for patients.
By leveraging digital platforms, researchers can manage patient recruitment, data entry, and follow-up more effectively. This efficient management translates into a better allocation of resources, allowing for wider participation and potentially including more diverse patient populations, thereby improving the generalizability of trial findings.
Enhanced Patient Engagement
Digital health platforms provide patients with convenient access to their health information and treatment plans, fostering greater engagement in their own care. This heightened level of engagement is crucial for adherence to treatment protocols and for providing valuable real-time feedback that can inform clinical decision-making. Increased patient engagement also positively impacts the overall quality and reliability of the data collected during clinical trials.
Furthermore, these platforms can empower patients to actively participate in their treatment journeys, leading to a greater sense of ownership and control over their health. This positive patient experience is a key factor in enhancing recruitment rates and ultimately improving the success of clinical trials.
Accelerated Regulatory Approvals
Digitalization of clinical trial data and documentation can facilitate a more efficient and transparent regulatory process. The streamlined submission of data, coupled with enhanced data integrity, allows regulatory agencies to process applications more quickly and effectively. This acceleration is vital in bringing new therapies to patients who need them, and it also fosters trust in the integrity of the entire process.
The use of standardized digital platforms and data formats can also help ensure data quality and consistency across different trials and jurisdictions, thereby facilitating smoother regulatory approvals across the globe.
Improved Data Quality and Integrity
Digital tools can significantly enhance the quality and integrity of clinical trial data by automating data entry, reducing human error, and implementing robust validation checks. This meticulous approach minimizes the risk of inaccuracies and inconsistencies, leading to more reliable and trustworthy results. The ability to meticulously track and audit data throughout the trial process is a critical component of transparency and reliability.
Personalized Medicine and Treatment Strategies
Digital health technologies enable the collection and analysis of vast amounts of patient data, providing valuable insights into individual patient characteristics and responses to treatment. This personalized approach can lead to the development of more effective and targeted therapies, tailoring treatments to specific patient needs and improving outcomes. The ability to track individual patient responses in real-time provides critical data for adjusting treatment plans and optimizing patient care.
Reduced Costs and Increased Efficiency
Streamlined clinical trials and regulatory processes driven by digital health solutions can lead to significant cost reductions. Automation of tasks, reduced administrative overhead, and improved data management contribute to a more efficient use of resources. These cost savings can be reinvested in research and development, ultimately accelerating the pace of innovation and improving patient access to cutting-edge therapies.
Addressing Ethical Considerations and Data Privacy
As digital health technologies become more integrated into clinical trials and regulatory processes, it is crucial to address the ethical implications and ensure data privacy and security. Robust data protection measures and ethical guidelines are essential to build trust and maintain patient confidentiality. The implementation of strict data governance frameworks and the adherence to ethical principles are paramount in ensuring the responsible and ethical use of digital health technologies in the healthcare sector. This fosters public trust and underscores the importance of maintaining the highest ethical standards in research and development.