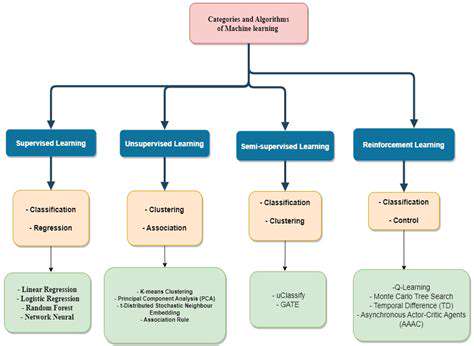
Beyond the Cas9 Scissors: Zinc Finger Nucleases (ZFNs)
Zinc finger nucleases (ZFNs) represent an early form of engineered gene editing tools. These proteins, designed to target specific DNA sequences, work by creating a double-strand break at the desired location. This break triggers the cell's natural DNA repair mechanisms, allowing for precise alterations. While ZFNs have demonstrated their potential in research settings, challenges in their design and delivery have hindered their widespread adoption in clinical applications. Scientists are continually refining these technologies to improve their efficiency and reduce potential off-target effects.
A key advantage of ZFNs is their modular design, allowing for the creation of specific targeting sequences. However, the laborious and expensive process of designing and testing ZFNs remains a significant hurdle, particularly for targeting complex genetic variations, a frequent component of rare genetic diseases. Scientists are actively exploring alternative approaches to streamline the development and application of ZFN technology.
TALENs: Transcription Activator-Like Effector Nucleases
Transcription activator-like effector nucleases (TALENs) offer a more streamlined approach to gene editing compared to ZFNs. TALENs, similar to ZFNs, are engineered proteins that can be directed to specific DNA sequences and induce a double-strand break. Their design is based on naturally occurring bacterial proteins, making them potentially more accessible to modify than ZFNs. This modular design simplifies the process of engineering new TALENs for different target sites.
A critical advantage of TALENs is their reduced off-target effects compared to ZFNs. This is a crucial consideration in any gene editing technology aiming for therapeutic applications. However, the delivery of TALENs into target cells remains a significant challenge, requiring further research to improve their efficiency.
Prime Editing: A Novel Approach to Gene Editing
Prime editing, a more recent advancement in gene editing, offers a unique approach to correcting genetic errors. Instead of simply creating a double-strand break, prime editing utilizes a prime editor protein complex to introduce precise changes to the DNA sequence. This method allows for base-pair replacements, insertions, and deletions without the need for a DNA repair template, making it a powerful tool for treating diverse genetic diseases. These improvements in precision and efficiency represent a significant step forward in gene therapy.
The ability to correct mutations directly without relying on the cell's repair mechanisms is a key strength of prime editing. This bypasses the potential for errors introduced during the cell's repair process, thus minimizing off-target effects. Further research into the efficiency and safety of prime editing is vital for its potential application in clinical settings.
Base Editing: Altering DNA Letters Directly
Base editing tools, a promising development in gene editing, offer a targeted approach to changing single DNA bases. Instead of creating a double-strand break, base editors modify the DNA sequence directly, minimizing the risks associated with the cell's error-prone repair mechanisms. This precision enables the correction of specific mutations without the potential for unwanted changes in the surrounding DNA sequence. This targeted approach is a significant advancement in gene editing technology.
Base editors can introduce specific changes to the DNA sequence, addressing a critical weakness of traditional gene editing techniques. They have shown great promise in correcting disease-causing mutations in cells and animal models, but further research is needed to explore their broader clinical applications and potential challenges, such as off-target effects and delivery methods.
Meganucleases: Targeting Large DNA Sequences
Meganucleases, engineered proteins that target larger DNA sequences, offer a unique approach to gene editing. These proteins can recognize and cleave specific DNA sequences that are more extensive than those targeted by ZFNs, TALENs, or CRISPR-Cas9. This capability is particularly advantageous for targeting complex genetic rearrangements or large gene deletions or insertions. This unique approach holds significant promise for treating genetic disorders involving larger genomic alterations.
Meganucleases offer a powerful tool to precisely target and modify larger DNA segments, making them potentially useful for treating disorders linked to structural variations in the genome. However, significant challenges remain in designing, producing, and delivering these complex protein constructs effectively, as well as confirming their safety and efficacy.